Gas and light: triggers of c-di-GMP-mediated regulation
- PMID: 37339911
- PMCID: PMC10505747
- DOI: 10.1093/femsre/fuad034
Gas and light: triggers of c-di-GMP-mediated regulation
Abstract
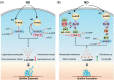
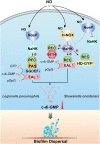
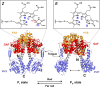
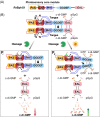
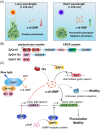
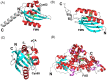
The widespread bacterial second messenger c-di-GMP is responsible for regulating many important physiological functions such as biofilm formation, motility, cell differentiation, and virulence. The synthesis and degradation of c-di-GMP in bacterial cells depend, respectively, on diguanylate cyclases and c-di-GMP-specific phosphodiesterases. Since c-di-GMP metabolic enzymes (CMEs) are often fused to sensory domains, their activities are likely controlled by environmental signals, thereby altering cellular c-di-GMP levels and regulating bacterial adaptive behaviors. Previous studies on c-di-GMP-mediated regulation mainly focused on downstream signaling pathways, including the identification of CMEs, cellular c-di-GMP receptors, and c-di-GMP-regulated processes. The mechanisms of CME regulation by upstream signaling modules received less attention, resulting in a limited understanding of the c-di-GMP regulatory networks. We review here the diversity of sensory domains related to bacterial CME regulation. We specifically discuss those domains that are capable of sensing gaseous or light signals and the mechanisms they use for regulating cellular c-di-GMP levels. It is hoped that this review would help refine the complete c-di-GMP regulatory networks and improve our understanding of bacterial behaviors in changing environments. In practical terms, this may eventually provide a way to control c-di-GMP-mediated bacterial biofilm formation and pathogenesis in general.
Keywords: NO sensors; c-di-GMP; c-di-GMP-specific phosphodiesterase; diguanylate cyclase; photoreceptors; sensory domains.
© The Author(s) 2023. Published by Oxford University Press on behalf of FEMS.
Conflict of interest statement
None declared.
Figures
References
-
- Abreu IA, Lourenço AI, Xavier AV et al. A novel iron centre in the split-soret cytochrome c from Desulfovibrio desulfuricans ATCC 27774. J Biol Inorg Chem. 2003;8:360–70. - PubMed
-
- Agari Y, Kashihara A, Yokoyama S et al. Global gene expression mediated by Thermus thermophilus SdrP, a CRP/FNR family transcriptional regulator. Mol Microbiol. 2008;70:60–75. - PubMed

