A platform trial of neoadjuvant and adjuvant antitumor vaccination alone or in combination with PD-1 antagonist and CD137 agonist antibodies in patients with resectable pancreatic adenocarcinoma
- PMID: 37339979
- PMCID: PMC10281953
- DOI: 10.1038/s41467-023-39196-9
A platform trial of neoadjuvant and adjuvant antitumor vaccination alone or in combination with PD-1 antagonist and CD137 agonist antibodies in patients with resectable pancreatic adenocarcinoma
Abstract
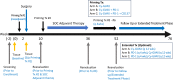
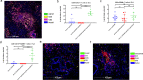
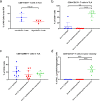
A neoadjuvant immunotherapy platform clinical trial allows for rapid evaluation of treatment-related changes in tumors and identifying targets to optimize treatment responses. We enrolled patients with resectable pancreatic adenocarcinoma into such a platform trial (NCT02451982) to receive pancreatic cancer GVAX vaccine with low-dose cyclophosphamide alone (Arm A; n = 16), with anti-PD-1 antibody nivolumab (Arm B; n = 14), and with both nivolumab and anti-CD137 agonist antibody urelumab (Arm C; n = 10), respectively. The primary endpoint for Arms A/B - treatment-related change in IL17A expression in vaccine-induced lymphoid aggregates - was previously published. Here, we report the primary endpoint for Arms B/C: treatment-related change in intratumoral CD8+ CD137+ cells and the secondary outcomes including safety, disease-free and overall survivals for all Arms. Treatment with GVAX+nivolumab+urelumab meets the primary endpoint by significantly increasing intratumoral CD8+ CD137+ cells (p = 0.003) compared to GVAX+Nivolumab. All treatments are well-tolerated. Median disease-free and overall survivals, respectively, are 13.90/14.98/33.51 and 23.59/27.01/35.55 months for Arms A/B/C. GVAX+nivolumab+urelumab demonstrates numerically-improved disease-free survival (HR = 0.55, p = 0.242; HR = 0.51, p = 0.173) and overall survival (HR = 0.59, p = 0.377; HR = 0.53, p = 0.279) compared to GVAX and GVAX+nivolumab, respectively, although not statistically significant due to small sample size. Therefore, neoadjuvant and adjuvant GVAX with PD-1 blockade and CD137 agonist antibody therapy is safe, increases intratumoral activated, cytotoxic T cells, and demonstrates a potentially promising efficacy signal in resectable pancreatic adenocarcinoma that warrants further study.
© 2023. The Author(s).
Conflict of interest statement
L.Z. receives grant support from Bristol-Meyer Squibb, Merck, Astrazeneca, iTeos, Amgen, NovaRock, Inxmed, and Halozyme. L.Z. is a paid consultant/Advisory Board Member at Biosion, Alphamab, NovaRock, Ambrx, Akrevia/Xilio, QED, Natera, Novagenesis, Snow Lake Capitals, BioArdis, Amberstone Biosciences, Tempus, Pfizer, Tavotek Lab, ClinicalTrial Options, LLC, and Mingruizhiyao. L.Z. holds shares at Alphamab, Amberstone, and Mingruizhiyao. E.J. reports other support from Abmeta and Adventris, personal fees from Achilles, Dragonfly, Mestag, The Medical Home Group, and Surgtx, other support from Parker Institute, grants and other support from the Lustgarten Foundation, Genentech, BMS, and Break Through Cancer outside the submitted work. D.T.L. serves on advisory boards for Merck, Bristol Myers Squibb, Nouscom, G1 Therapeutics, Janssen, and Merus and has received research funding from Merck, Bristol Myers Squibb, Curegenix, Nouscom, and Abbvie. She has received speaking honoraria from Merck and is an inventor of licensed intellectual property related to technology for mismatch repair deficiency for diagnosis and therapy (WO2016077553A1) from Johns Hopkins University. The terms of these arrangements are being managed by Johns Hopkins. R.A. receives grant support from Bristol-Meyer Squibb, RAPT Theraputics. R.A. is a paid consultant for Bristol-Meyer Squibb, Merck, Astrazeneca. The remaining authors declare no other competing interests.
Figures


References
-
- Sohal DPS, et al. Metastatic pancreatic cancer: ASCO guideline update. J. Clin. Oncol. 38, JCO2001364 (2020). - PubMed
-
- Laheru D, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin. Cancer Res. 2008;14:1455–1463. doi: 10.1158/1078-0432.CCR-07-0371. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

