Booster effect of the third dose of SARS-CoV-2 mRNA vaccine in Japanese kidney transplant recipients
- PMID: 37340001
- PMCID: PMC10281951
- DOI: 10.1038/s41598-023-36998-1
Booster effect of the third dose of SARS-CoV-2 mRNA vaccine in Japanese kidney transplant recipients
Abstract
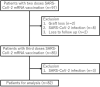
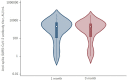
The humoral response of kidney transplant recipients (KTR) to the mRNA vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is generally poor. We evaluated the booster effect of the third dose (D3) of two SARS-CoV-2 mRNA vaccines 6 months after the second dose (D2) in Japanese KTR. The anti-spike (anti-S) antibody titer 1 and 3 months after the D3 was evaluated in 82 Japanese KTR. The primary endpoint was the seropositivity rate, and factors associated with the lack of a response were evaluated in a logistic regression model. Overall, the anti-S antibody seropositivity rate 1 and 3 months after the D3 was 74.7% and 76.0%. The anti-S antibody titers after the first and second doses were higher in patients vaccinated with the mRNA-1273 than with the BNT162b2 vaccine. Among the 38 KTR who were seronegative 5 months after the D2, 18 (47.4%) became seropositive following the D3. Factors associated with a non-response were mycophenolic acid dose, post-transplant duration, hemoglobin, and lymphocyte count. A humoral response 1 and 3 months after the D3 was obtained in ~ 75% of KTR, but 20% were non-responders. Additional studies are needed to clarify the factors hindering a vaccine response.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Japanese Society for Transplantation COVID-19 Task Force. Available online at: https://square.umin.ac.jp/jst-covid-19/images/20220831covid-19cases.pdf. Accessed on 23th Nov 2022.
-
- Ministry of Health, Labour and Welfare. Available online at: https://www.mhlw.go.jp/content/10906000/000970173.pdf. Accessed on 23th Nov 2022.
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

