CHI3L1 induces autophagy through the JNK pathway in lung cancer cells
- PMID: 37340009
- PMCID: PMC10281972
- DOI: 10.1038/s41598-023-36844-4
CHI3L1 induces autophagy through the JNK pathway in lung cancer cells
Abstract
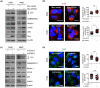
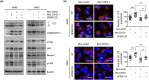
CHI3L1 is closely related to the molecular mechanisms of cancer cell migration, growth, and death. According to recent research, autophagy regulates tumor growth during various stages of cancer development. This study examined the association between CHI3L1 and autophagy in human lung cancer cells. In CHI3L1-overexpressing lung cancer cells, the expression of LC3, an autophagosome marker, and the accumulation of LC3 puncta increased. In contrast, CHI3L1 depletion in lung cancer cells decreased the formation of autophagosomes. Additionally, CHI3L1 overexpression promoted the formation of autophagosomes in various cancer cell lines: it also increased the co-localization of LC3 and the lysosome marker protein LAMP-1, indicating an increase in the production of autolysosomes. In mechanism study, CHI3L1 promotes autophagy via activation of JNK signaling. JNK may be crucial for CHI3L1-induced autophagy since pretreatment with the JNK inhibitor reduced the autophagic effect. Consistent with the in vitro model, the expression of autophagy-related proteins was downregulated in the tumor tissues of CHI3L1-knockout mice. Furthermore, the expression of autophagy-related proteins and CHI3L1 increased in lung cancer tissues compared with normal lung tissues. These findings show that CHI3L1-induced autophagy is triggered by JNK signals and that CHI3L1-induced autophagy could be a novel therapeutic approach to lung cancer.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

