Use of Electronic Health Record Data for Drug Safety Signal Identification: A Scoping Review
- PMID: 37340238
- PMCID: PMC11635839
- DOI: 10.1007/s40264-023-01325-0
Use of Electronic Health Record Data for Drug Safety Signal Identification: A Scoping Review
Abstract
Introduction: Pharmacovigilance programs protect patient health and safety by identifying adverse event signals through postmarketing surveillance of claims data and spontaneous reports. Electronic health records (EHRs) provide new opportunities to address limitations of traditional approaches and promote discovery-oriented pharmacovigilance.
Methods: To evaluate the current state of EHR-based medication safety signal identification, we conducted a scoping literature review of studies aimed at identifying safety signals from routinely collected patient-level EHR data. We extracted information on study design, EHR data elements utilized, analytic methods employed, drugs and outcomes evaluated, and key statistical and data analysis choices.
Results: We identified 81 eligible studies. Disproportionality methods were the predominant analytic approach, followed by data mining and regression. Variability in study design makes direct comparisons difficult. Studies varied widely in terms of data, confounding adjustment, and statistical considerations.
Conclusion: Despite broad interest in utilizing EHRs for safety signal identification, current efforts fail to leverage the full breadth and depth of available data or to rigorously control for confounding. The development of best practices and application of common data models would promote the expansion of EHR-based pharmacovigilance.
© 2023. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
Statements and Declarations
Figures


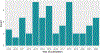
References
-
- World Health Organization. What is Pharmacovigilance? [Internet]. [cited 2022 Jul 22]. Available from: https://www.who.int/teams/regulation-prequalification/regulation-and-saf...
-
- Hauben M, Aronson JK. Defining “signal” and its subtypes in pharmacovigilance based on a systematic review of previous definitions. Drug Saf. 2009;32:99–110. - PubMed
-
- Coloma PM, Trifiro G, Patadia V, Sturkenboom M Postmarketing safety surveillance: Where does signal detection using electronic healthcare records fit into the big picture? Drug Saf. 2013;36:183–97. - PubMed
-
- U.S. Food & Drug Administration. Questions and Answers on FDA’s Adverse Event Reporting System (FAERS) [Internet]. 2018. Available from: https://www.fda.gov/drugs/surveillance/questions-and-answers-fdas-advers...
-
- European Medicines Agency. EudraVigilance system overview [Internet]. [cited 2022 Aug 25]. Available from: https://www.ema.europa.eu/en/human-regulatory/research-development/pharm...
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

