A randomized controlled pilot trial to assess the effectiveness of a specially formulated food supplement and pelvic floor muscle training in women with stress-predominant urinary incontinence
- PMID: 37340306
- PMCID: PMC10283266
- DOI: 10.1186/s12905-023-02476-z
A randomized controlled pilot trial to assess the effectiveness of a specially formulated food supplement and pelvic floor muscle training in women with stress-predominant urinary incontinence
Abstract
Background: Pelvic floor muscle training (PFMT) is the first-line treatment approach for stress urinary incontinence. Creatine and leucine have been shown to improve muscle function. Our aim was to assess the effectiveness of a food supplement and PFMT in women with stress-predominant urinary incontinence.
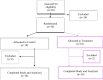
Methods: Women with stress-predominant urinary incontinence were randomized in 1:1 ratio to receive daily oral supplementation for six weeks with either a food supplement (treatment group) or placebo (control group). Both groups were instructed to perform standardized daily PFMT. The primary outcome was the Urogenital Distress Inventory Short Form (UDI-6) score. Secondary outcomes were the Incontinence Impact Questionnaire (IIQ-7) score, Patient's Global Impression of Severity (PGI-S), and Biomechanical Integrity score (BI-score) measured by Vaginal Tactile Imager. To have a power of 80% and a significance level of 5% to detect a decrease of 16 points in the UDI-6 score, a sample size of 32 was needed, with 16 patients in each arm of our trial.
Results: Sixteen women in the control group and sixteen in the treatment group completed the trial. Between-group analysis revealed no significant differences between the control and treatment group except for mean change (delta) in vaginal squeeze pressure [(cmH2O, mean ± SD), 5 ± 12 vs. 15 ± 15, P = 0.04] and mean change (delta) in PGI-S score [(mean ± SD), -0.2 ± 0.9 vs. -0.8 ± 0.8, P = 0.04]. Within-group analysis showed that UDI-6 and IIQ-7 scores improved significantly from baseline to six weeks in the treatment group but not in the control group [UDI-6 score (mean ± SD) 45 ± 21 vs. 29 ± 21, P = 0.02; 43 ± 18 vs. 33 ± 26, P = 0.22] [IIQ-7 score (mean ± SD) 50 ± 30 vs. 30 ± 21, P = 0.01; 48 ± 23 vs.40 ± 28, P = 0.36]. PGI-S scores only improved in the treatment group from baseline to six weeks after treatment [PGI-S score (mean ± SD) 3.1 ± 0.8 vs. 2.3 ± 0.8, P = 0.0001]. BI-score, on average, improved significantly in the treatment and control group as well [SD unit, mean, from - 1.06 to -0.58, P = 0.001; from - 0.66 to -0.42, P = 0.04].
Conclusions: Women with stress-predominant urinary incontinence receiving a specially formulated supplement in addition to daily PFMT for six weeks had significantly improved urinary symptoms (decrease in UDI-6 score and IIQ-7) and BI-score compared to their baseline.
Trial registration: ClinicalTrials.gov Identifier: NCT05358769. 27/04/2022.
Keywords: Creatine; Leucine; Pelvic floor; Urinary incontinence; Zinc.
© 2023. The Author(s).
Conflict of interest statement
PT is a paid consultant for Fempharma LLC. All the other co-authors declared no conflict of interest.
Figures

Similar articles
-
The impact of short-term pelvic floor muscle training on the biomechanical parameters of the pelvic floor among patients with stress urinary incontinence: A pilot study.Eur J Obstet Gynecol Reprod Biol. 2024 Nov;302:283-287. doi: 10.1016/j.ejogrb.2024.09.037. Epub 2024 Sep 25. Eur J Obstet Gynecol Reprod Biol. 2024. PMID: 39348760
-
Evaluation of the effect of pelvic floor muscle training (PFMT or Kegel exercise) and assisted pelvic floor muscle training (APFMT) by a resistance device (Kegelmaster device) on the urinary incontinence in women: a randomized trial.Eur J Obstet Gynecol Reprod Biol. 2011 Nov;159(1):218-23. doi: 10.1016/j.ejogrb.2011.06.037. Epub 2011 Jul 7. Eur J Obstet Gynecol Reprod Biol. 2011. PMID: 21741151 Clinical Trial.
-
Randomized trial comparing efficacy of pelvic floor muscle training with a digital therapeutic motion-based device to standard pelvic floor exercises for treatment of stress urinary incontinence (SUV trial): An all-virtual trial design.Contemp Clin Trials. 2021 Jun;105:106406. doi: 10.1016/j.cct.2021.106406. Epub 2021 Apr 16. Contemp Clin Trials. 2021. PMID: 33866003 Clinical Trial.
-
Effect of pelvic floor muscle training using mobile health applications for stress urinary incontinence in women: a systematic review.BMC Womens Health. 2022 Oct 3;22(1):400. doi: 10.1186/s12905-022-01985-7. BMC Womens Health. 2022. PMID: 36192744 Free PMC article.
-
Commercially Available Home Pelvic Training Devices for the Treatment of Pelvic Floor Disorders: A Systematic Review and Meta-analysis.Obstet Gynecol. 2022 Aug 1;140(2):275-292. doi: 10.1097/AOG.0000000000004860. Epub 2022 Jul 6. Obstet Gynecol. 2022. PMID: 35852280
Cited by
-
Association between dietary intake of creatine and female reproductive health: Evidence from NHANES 2017-2020.Food Sci Nutr. 2024 Apr 30;12(7):4893-4898. doi: 10.1002/fsn3.4135. eCollection 2024 Jul. Food Sci Nutr. 2024. PMID: 39055234 Free PMC article.
-
Comprehensive Analysis of the Biomechanical Research of Pelvic Organ Prolapse: A Scientometric Approach.J Multidiscip Healthc. 2025 Mar 1;18:1249-1268. doi: 10.2147/JMDH.S473196. eCollection 2025. J Multidiscip Healthc. 2025. PMID: 40046658 Free PMC article.
References
-
- Bo K, Frawley HC, Haylen BT, Abramov Y, Almeida FG, Berghmans B et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the conservative and nonpharmacological management of female pelvic floor dysfunction. Int Urogynecol J 2017 Feb;28(2):191–213. - PubMed
-
- Myers DL. Female mixed urinary incontinence: a clinical review. JAMA 2014 May 21;311(19):2007–2014. - PubMed
-
- Kinchen KS, Lee J, Fireman B, Hunkeler E, Nehemiah JL, Curtice TG. The prevalence, burden, and treatment of urinary incontinence among women in a managed care plan. J Womens Health (Larchmt). 2007 Apr;16(3):415–22. - PubMed
-
- Morrison A, Levy R. Fraction of nursing home admissions attributable to urinary incontinence. Value Health 2006 Jul-Aug;9(4):272–4. - PubMed
-
- Nambiar AK, Arlandis S, Bo K, Cobussen-Boekhorst H, Costantini E, de Heide M, et al. European Association of Urology Guidelines on the diagnosis and management of female non-neurogenic lower urinary tract symptoms. Part 1: Diagnostics, overactive bladder, stress urinary incontinence, and mixed urinary incontinence. Eur Urol. 2022 Jul;82(1):49–59. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

