NIPSNAP1 directs dual mechanisms to restrain senescence in cancer cells
- PMID: 37340421
- PMCID: PMC10280965
- DOI: 10.1186/s12967-023-04232-1
NIPSNAP1 directs dual mechanisms to restrain senescence in cancer cells
Abstract
Background: Although the executive pathways of senescence are known, the underlying control mechanisms are diverse and not fully understood, particularly how cancer cells avoid triggering senescence despite experiencing exacerbated stress conditions within the tumor microenvironment.
Methods: Mass spectrometry (MS)-based proteomic screening was used to identify differentially regulated genes in serum-starved hepatocellular carcinoma cells and RNAi employed to determine knockdown phenotypes of prioritized genes. Thereafter, gene function was investigated using cell proliferation assays (colony-formation, CCK-8, Edu incorporation and cell cycle) together with cellular senescence assays (SA-β-gal, SAHF and SASP). Gene overexpression and knockdown techniques were applied to examine mRNA and protein regulation in combination with luciferase reporter and proteasome degradation assays, respectively. Flow cytometry was applied to detect changes in cellular reactive oxygen species (ROS) and in vivo gene function examined using a xenograft model.
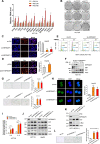
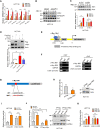
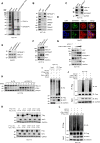
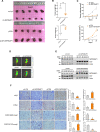
Results: Among the genes induced by serum deprivation, NIPSNAP1 was selected for investigation. Subsequent experiments revealed that NIPSNAP1 promotes cancer cell proliferation and inhibits P27-dependent induction of senescence via dual mechanisms. Firstly, NIPSNAP1 maintains the levels of c-Myc by sequestering the E3 ubiquitin ligase FBXL14 to prevent the proteasome-mediated turnover of c-Myc. Intriguingly, NIPSNAP1 levels are restrained by transcriptional repression mediated by c-Myc-Miz1, with repression lifted in response to serum withdrawal, thus identifying feedback regulation between NIPSNAP1 and c-Myc. Secondly, NIPSNAP1 was shown to modulate ROS levels by promoting interactions between the deacetylase SIRT3 and superoxide dismutase 2 (SOD2). Consequent activation of SOD2 serves to maintain cellular ROS levels below the critical levels required to induce cell cycle arrest and senescence. Importantly, the actions of NIPSNAP1 in promoting cancer cell proliferation and preventing senescence were recapitulated in vivo using xenograft models.
Conclusions: Together, these findings reveal NIPSNAP1 as an important mediator of c-Myc function and a negative regulator of cellular senescence. These findings also provide a theoretical basis for cancer therapy where targeting NIPSNAP1 invokes cellular senescence.
Keywords: Acetylation; Cellular senescence; FBXL14; NIPSNAP1; ROS; SIRT3; SOD2; c-Myc.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Inhibition of the FEN1-PBX1 axis elicits cellular senescence in breast cancer via the increased intracellular reactive oxygen species levels.J Transl Med. 2025 Feb 28;23(1):248. doi: 10.1186/s12967-025-06216-9. J Transl Med. 2025. PMID: 40022092 Free PMC article.
-
Knockdown of inwardly rectifying potassium channel Kir2.2 suppresses tumorigenesis by inducing reactive oxygen species-mediated cellular senescence.Mol Cancer Ther. 2010 Nov;9(11):2951-9. doi: 10.1158/1535-7163.MCT-10-0511. Epub 2010 Sep 14. Mol Cancer Ther. 2010. PMID: 20841375
-
Ionizing radiation induces cellular senescence of articular chondrocytes via negative regulation of SIRT1 by p38 kinase.J Biol Chem. 2010 Jan 8;285(2):1283-95. doi: 10.1074/jbc.M109.058628. Epub 2009 Nov 3. J Biol Chem. 2010. PMID: 19887452 Free PMC article.
-
Transcriptional Heterogeneity of Cellular Senescence in Cancer.Mol Cells. 2022 Sep 30;45(9):610-619. doi: 10.14348/molcells.2022.0036. Epub 2022 Aug 19. Mol Cells. 2022. PMID: 35983702 Free PMC article. Review.
-
The underlying mechanism of nuclear and mitochondrial DNA damages in triggering cancer incidences: Insights into proteomic and genomic sciences.J Biotechnol. 2024 Mar 10;383:1-12. doi: 10.1016/j.jbiotec.2024.01.013. Epub 2024 Feb 2. J Biotechnol. 2024. PMID: 38309588 Review.
Cited by
-
Mitochondrial Physiology of Cellular Redox Regulations.Physiol Res. 2024 Aug 30;73(S1):S217-S242. doi: 10.33549/physiolres.935269. Epub 2024 Apr 22. Physiol Res. 2024. PMID: 38647168 Free PMC article. Review.
-
N6-methyladenosine reader IGF2BP2 regulates NIPSNAP1-mediated mitophagy and mitochondrial dynamics to alleviate hepatic ischemia-reperfusion injury.World J Gastroenterol. 2025 Jun 14;31(22):105157. doi: 10.3748/wjg.v31.i22.105157. World J Gastroenterol. 2025. PMID: 40539202 Free PMC article.
-
Advanced Proteomics Approaches Hold Potential for the Risk Assessment of Metabolism-Disrupting Chemicals as Omics-Based NAM: A Case Study Using the Phthalate Substitute DINCH.Environ Sci Technol. 2025 Aug 12;59(31):16193-16216. doi: 10.1021/acs.est.5c01206. Epub 2025 Jul 30. Environ Sci Technol. 2025. PMID: 40736767 Free PMC article.
-
Inhibition of the FEN1-PBX1 axis elicits cellular senescence in breast cancer via the increased intracellular reactive oxygen species levels.J Transl Med. 2025 Feb 28;23(1):248. doi: 10.1186/s12967-025-06216-9. J Transl Med. 2025. PMID: 40022092 Free PMC article.
-
Role of mitophagy in spinal cord ischemia-reperfusion injury.Neural Regen Res. 2026 Feb 1;21(2):598-611. doi: 10.4103/NRR.NRR-D-24-00668. Epub 2024 Dec 7. Neural Regen Res. 2026. PMID: 39665804 Free PMC article.
References
-
- Roninson IB. Tumor cell senescence in cancer treatment. Cancer Res. 2003;63:2705–2715. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

