Self-healing hydrogel as an injectable implant: translation in brain diseases
- PMID: 37340481
- PMCID: PMC10280980
- DOI: 10.1186/s12929-023-00939-x
Self-healing hydrogel as an injectable implant: translation in brain diseases
Abstract
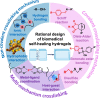
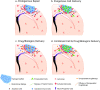
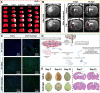
Tissue engineering biomaterials are aimed to mimic natural tissue and promote new tissue formation for the treatment of impaired or diseased tissues. Highly porous biomaterial scaffolds are often used to carry cells or drugs to regenerate tissue-like structures. Meanwhile, self-healing hydrogel as a category of smart soft hydrogel with the ability to automatically repair its own structure after damage has been developed for various applications through designs of dynamic crosslinking networks. Due to flexibility, biocompatibility, and ease of functionalization, self-healing hydrogel has great potential in regenerative medicine, especially in restoring the structure and function of impaired neural tissue. Recent researchers have developed self-healing hydrogel as drug/cell carriers or tissue support matrices for targeted injection via minimally invasive surgery, which has become a promising strategy in treating brain diseases. In this review, the development history of self-healing hydrogel for biomedical applications and the design strategies according to different crosslinking (gel formation) mechanisms are summarized. The current therapeutic progress of self-healing hydrogels for brain diseases is described as well, with an emphasis on the potential therapeutic applications validated by in vivo experiments. The most recent aspect as well as the design rationale of self-healing hydrogel for different brain diseases is also addressed.
Keywords: Injectable implant; Neural tissue engineering; Neurodegenerative disease; Self-healing hydrogel; Stroke; Translation medicine; Traumatic brain injury.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

