Pathological ultrastructural alterations of myelinated axons in normal appearing white matter in progressive multiple sclerosis
- PMID: 37340488
- PMCID: PMC10283269
- DOI: 10.1186/s40478-023-01598-7
Pathological ultrastructural alterations of myelinated axons in normal appearing white matter in progressive multiple sclerosis
Abstract
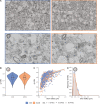
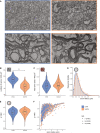
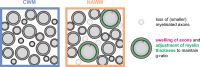
Multiple sclerosis (MS) pathophysiology includes inflammation, demyelination and neurodegeneration, but the exact mechanisms of disease initiation and progression are unknown. A major feature of lesions is lack of myelin, which increases axonal energy demand and requires adaptation in number and size of mitochondria. Outside lesions, subtle and diffuse alterations are observed in normal appearing white matter (NAWM) and normal appearing grey matter (NAGM), including increased oxidative stress, reduced axon density and changes in myelin composition and morphology. On an ultrastructural level, only limited data is available on alterations in myelinated axons. We generated large scale 2D scanning transmission electron microscopy images ('nanotomy') of non-demyelinated brain tissue of control and progressive MS donors, accessible via an open-access online repository. We observed a reduced density of myelinated axons in NAWM, without a decrease in cross-sectional axon area. Small myelinated axons were less frequently and large myelinated axons were more frequently present in NAWM, while the g-ratio was similar. The correlation between axonal mitochondrial radius and g-ratio was lost in NAWM, but not in NAGM. Myelinated axons in control GM and NAGM had a similar g-ratio and radius distribution. We hypothesize that axonal loss in NAWM is likely compensated by swelling of the remaining myelinated axons and subsequent adjustment of myelin thickness to maintain their g-ratio. Failure of axonal mitochondria to adjust their size and fine-tuning of myelin thickness may render NAWM axons and their myelin more susceptible to injury.
Keywords: Electron microscopy; Human brain (bank); Mitochondria; Multiple sclerosis; Myelin; Nanotomy; Ultrastructure; g-ratio.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Ramió-Torrentà L, Sastre-Garriga J, Ingle GT, et al. Abnormalities in normal appearing tissues in early primary progressive multiple sclerosis and their relation to disability: a tissue specific magnetisation transfer study. J Neurol Neurosurg Psychiatry. 2006;77:40. doi: 10.1136/JNNP.2004.052316. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

