Smoking and tetramer tryptase accelerate intervertebral disc degeneration by inducing METTL14-mediated DIXDC1 m6 modification
- PMID: 37340635
- PMCID: PMC10422004
- DOI: 10.1016/j.ymthe.2023.06.010
Smoking and tetramer tryptase accelerate intervertebral disc degeneration by inducing METTL14-mediated DIXDC1 m6 modification
Abstract
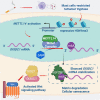
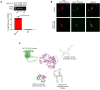
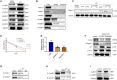
Although cigarette smoking (CS) and low back pain (LBP) are common worldwide, their correlations and the mechanisms of action remain unclear. We have shown that excessive activation of mast cells (MCs) and their proteases play key roles in CS-associated diseases, like asthma, chronic obstructive pulmonary disease (COPD), blood coagulation, and lung cancer. Previous studies have also shown that MCs and their proteases induce degenerative musculoskeletal disease. By using a custom-designed smoke-exposure mouse system, we demonstrated that CS results in intervertebral disc (IVD) degeneration and release of MC-restricted tetramer tryptases (TTs) in the IVDs. TTs were found to regulate the expression of methyltransferase 14 (METTL14) at the epigenetic level by inducing N6-methyladenosine (m6A) deposition in the 3' untranslated region (UTR) of the transcript that encodes dishevelled-axin (DIX) domain-containing 1 (DIXDC1). That reaction increases the mRNA stability and expression of Dixdc1. DIXDC1 functionally interacts with disrupted in schizophrenia 1 (DISC1) to accelerate the degeneration and senescence of nucleus pulposus (NP) cells by activating a canonical Wnt pathway. Our study demonstrates the association between CS, MC-derived TTs, and LBP. These findings raise the possibility that METTL14-medicated DIXDC1 m6A modification could serve as a potential therapeutic target to block the development of degeneration of the NP in LBP patients.
Keywords: intervertebral disc; low back pain; mast cells; smoking; spine.
Copyright © 2023 The American Society of Gene and Cell Therapy. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





Similar articles
-
m6A hypomethylation of DNMT3B regulated by ALKBH5 promotes intervertebral disc degeneration via E4F1 deficiency.Clin Transl Med. 2022 Mar;12(3):e765. doi: 10.1002/ctm2.765. Clin Transl Med. 2022. PMID: 35340126 Free PMC article.
-
CCN family member 2/connective tissue growth factor (CCN2/CTGF) is regulated by Wnt-β-catenin signaling in nucleus pulposus cells.Arthritis Res Ther. 2018 Sep 29;20(1):217. doi: 10.1186/s13075-018-1723-8. Arthritis Res Ther. 2018. PMID: 30268161 Free PMC article.
-
Mast Cell-Intervertebral disc cell interactions regulate inflammation, catabolism and angiogenesis in Discogenic Back Pain.Sci Rep. 2017 Oct 2;7(1):12492. doi: 10.1038/s41598-017-12666-z. Sci Rep. 2017. PMID: 28970490 Free PMC article.
-
M6A methylation-regulated autophagy may be a new therapeutic target for intervertebral disc degeneration.Cell Biol Int. 2024 Apr;48(4):389-403. doi: 10.1002/cbin.12135. Epub 2024 Feb 5. Cell Biol Int. 2024. PMID: 38317355 Review.
-
Bioenergetic dysfunction in the pathogenesis of intervertebral disc degeneration.Pharmacol Res. 2024 Apr;202:107119. doi: 10.1016/j.phrs.2024.107119. Epub 2024 Feb 28. Pharmacol Res. 2024. PMID: 38417775 Review.
Cited by
-
Global burden of low back pain attributable to smoking in 204 countries and territories in 1990-2021.Front Public Health. 2025 Jul 10;13:1584659. doi: 10.3389/fpubh.2025.1584659. eCollection 2025. Front Public Health. 2025. PMID: 40709036 Free PMC article.
-
The Current Molecular and Cellular Landscape of Chronic Obstructive Pulmonary Disease (COPD): A Review of Therapies and Efforts towards Personalized Treatment.Proteomes. 2024 Aug 16;12(3):23. doi: 10.3390/proteomes12030023. Proteomes. 2024. PMID: 39189263 Free PMC article. Review.
-
Mechanisms and Therapeutic Strategies of Macrophage Polarization in Intervertebral Disc Degeneration.JOR Spine. 2025 May 14;8(2):e70065. doi: 10.1002/jsp2.70065. eCollection 2025 Jun. JOR Spine. 2025. PMID: 40371270 Free PMC article. Review.
-
Risk factors for low back pain in the Chinese population: a systematic review and meta-analysis.BMC Public Health. 2024 Apr 26;24(1):1181. doi: 10.1186/s12889-024-18510-0. BMC Public Health. 2024. PMID: 38671417 Free PMC article.
-
REDOX Imbalance and Oxidative Stress in the Intervertebral Disc: The Effect of Mechanical Stress and Cigarette Smoking on ER Stress and Mitochondrial Dysfunction.Cells. 2025 Apr 19;14(8):613. doi: 10.3390/cells14080613. Cells. 2025. PMID: 40277939 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

