Culture media and format alter cellular composition and barrier integrity of porcine colonoid-derived monolayers
- PMID: 37340938
- PMCID: PMC11042055
- DOI: 10.1080/21688370.2023.2222632
Culture media and format alter cellular composition and barrier integrity of porcine colonoid-derived monolayers
Abstract
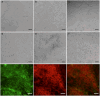
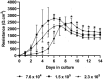
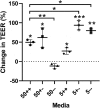
Intestinal organoid technology has revolutionized our approach to in vitro cell culture due in part to their three-dimensional structures being more like the native tissue from which they were derived with respect to cellular composition and architecture. For this reason, organoids are becoming the new gold standard for undertaking intestinal epithelial cell research. Unfortunately, their otherwise advantageous three-dimensional geometry prevents easy access to the apical epithelium, which is a major limitation when studying interactions between dietary or microbial components and host tissues. To overcome this problem, we developed porcine colonoid-derived monolayers cultured on both permeable Transwell inserts and tissue culture treated polystyrene plates. We found that seeding density and culture format altered the expression of genes encoding markers of specific cell types (stem cells, colonocytes, goblets, and enteroendocrine cells), and barrier maturation (tight junctions). Additionally, we found that changes to the formulation of the culture medium altered the cellular composition of colonoids and of monolayers derived from them, resulting in cultures with an increasingly differentiated phenotype that was similar to that of their tissue of origin.
Keywords: Barrier integrity; Porcine colonoids; colonoid-derived monolayers; differentiation; medium formulation; stem cells.
Plain language summary
In vitro models of the intestine are used to study the complex in vivo intestinal processes in a simplified context. As such, these models need to be representative of their tissue of origin. Here, we demonstrate that porcine colonoids and colonoid-derived monolayers that have comparable stem cells and differentiated cell types to those of the native tissue can be developed but are influenced by cell seeding density, culture format, and medium formulation.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures





Similar articles
-
Porcine colonoids and enteroids keep the memory of their origin during regeneration.Am J Physiol Cell Physiol. 2021 May 1;320(5):C794-C805. doi: 10.1152/ajpcell.00420.2020. Epub 2021 Mar 24. Am J Physiol Cell Physiol. 2021. PMID: 33760661
-
Drivers of transcriptional variance in human intestinal epithelial organoids.Physiol Genomics. 2021 Nov 1;53(11):486-508. doi: 10.1152/physiolgenomics.00061.2021. Epub 2021 Oct 6. Physiol Genomics. 2021. PMID: 34612061 Free PMC article.
-
Co-Culture System of Human Enteroids/Colonoids with Innate Immune Cells.Curr Protoc Immunol. 2020 Dec;131(1):e113. doi: 10.1002/cpim.113. Curr Protoc Immunol. 2020. PMID: 33166041 Free PMC article.
-
Intestinal organoids in farm animals.Vet Res. 2021 Feb 25;52(1):33. doi: 10.1186/s13567-021-00909-x. Vet Res. 2021. PMID: 33632315 Free PMC article. Review.
-
Brief summary of the current protocols for generating intestinal organoids.Dev Growth Differ. 2018 Aug;60(6):387-392. doi: 10.1111/dgd.12559. Epub 2018 Jul 23. Dev Growth Differ. 2018. PMID: 30039581 Review.
Cited by
-
Development and characterization of segment-specific enteroids from the pig small intestine in Matrigel and transwell inserts: insights into susceptibility to porcine epidemic diarrhea Virus.Front Immunol. 2024 Sep 17;15:1451154. doi: 10.3389/fimmu.2024.1451154. eCollection 2024. Front Immunol. 2024. PMID: 39355235 Free PMC article.
References
-
- Grossmann J, Walther K, Artinger M, Kiessling S, Steinkamp M, Schmautz WK, Stadler F, Bataille F, Schultz M, Schölmerich J, et al. Progress on isolation and short-term ex-vivo culture of highly purified non-apoptotic human intestinal epithelial cells (IEC). Eur J Cell Biol. 2003;82(5):262–270. doi:10.1078/0171-9335-00312. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
