Pharmacokinetic pharmacodynamic modeling of analgesics and sedatives in children
- PMID: 37341161
- PMCID: PMC10947261
- DOI: 10.1111/pan.14712
Pharmacokinetic pharmacodynamic modeling of analgesics and sedatives in children
Abstract
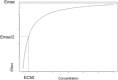
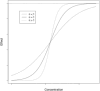
Pharmacokinetic pharmacodynamic modeling is an important tool which uses statistical methodology to provide a better understanding of the relationship between concentration and effect of drugs such as analgesics and sedatives. Pharmacokinetic pharmacodynamic models also describe between-subject variability that allows identification of subgroups and dose adjustment for optimal pain management in individual patients. This approach is particularly useful in the pediatric population, where most drugs have received limited evaluation and dosing is extrapolated from adult practice. In children, the covariates of weight and age are used to describe size- and maturation-related changes in pharmacokinetics. It is important to consider both size and maturation in order to develop an accurate model and determine the optimal dose for different age groups. An adequate assessment of analgesic and sedative effect using pain scales or brain activity measures is essential to build reliable pharmacokinetic pharmacodynamic models. This is often challenging in children due to the multidimensional nature of pain and the limited sensitivity and specificity of some measurement tools. This review provides a summary of the pharmacokinetic and pharmacodynamic methodology used to describe the dose-concentration-effect relationship of analgesics and sedation in children, with a focus on the different pharmacodynamic endpoints and the challenges of pharmacodynamic modeling.
Keywords: children; pain; pharmacodynamic; pharmacokinetic; sedation.
© 2023 The Authors. Pediatric Anesthesia published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors report no financial conflict of interest relevant to this manuscript. Suellen Walker is a Section Editor for
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

