Irreversible Inactivation of SARS-CoV-2 by Lectin Engagement with Two Glycan Clusters on the Spike Protein
- PMID: 37341186
- PMCID: PMC10663058
- DOI: 10.1021/acs.biochem.3c00109
Irreversible Inactivation of SARS-CoV-2 by Lectin Engagement with Two Glycan Clusters on the Spike Protein
Abstract
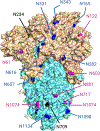
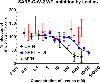
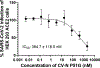
Host cell infection by SARS-CoV-2, similar to that by HIV-1, is driven by a conformationally metastable and highly glycosylated surface entry protein complex, and infection by these viruses has been shown to be inhibited by the mannose-specific lectins cyanovirin-N (CV-N) and griffithsin (GRFT). We discovered in this study that CV-N not only inhibits SARS-CoV-2 infection but also leads to irreversibly inactivated pseudovirus particles. The irreversibility effect was revealed by the observation that pseudoviruses first treated with CV-N and then washed to remove all soluble lectin did not recover infectivity. The infection inhibition of SARS-CoV-2 pseudovirus mutants with single-site glycan mutations in spike suggested that two glycan clusters in S1 are important for both CV-N and GRFT inhibition: one cluster associated with the RBD (receptor binding domain) and the second with the S1/S2 cleavage site. We observed lectin antiviral effects with several SARS-CoV-2 pseudovirus variants, including the recently emerged omicron, as well as a fully infectious coronavirus, therein reflecting the breadth of lectin antiviral function and the potential for pan-coronavirus inactivation. Mechanistically, observations made in this work indicate that multivalent lectin interaction with S1 glycans is likely a driver of the lectin infection inhibition and irreversible inactivation effect and suggest the possibility that lectin inactivation is caused by an irreversible conformational effect on spike. Overall, lectins' irreversible inactivation of SARS-CoV-2, taken with their breadth of function, reflects the therapeutic potential of multivalent lectins targeting the vulnerable metastable spike before host cell encounter.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
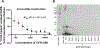
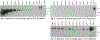
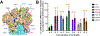
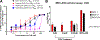
References
-
- Cai Y; Xu W; Gu C; Cai X; Qu D; Lu L; Xie Y; Jiang S Griffithsin with A Broad-Spectrum Antiviral Activity by Binding Glycans in Viral Glycoprotein Exhibits Strong Synergistic Effect in Combination with A Pan-Coronavirus Fusion Inhibitor Targeting SARS-CoV-2 Spike S2 Subunit. Virol. Sin. 2020, 35, 857–860. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

