Polyclonal anti-thymocyte globulins for the prophylaxis of graft-versus-host disease after allogeneic stem cell or bone marrow transplantation in adults
- PMID: 37341189
- PMCID: PMC10284458
- DOI: 10.1002/14651858.CD009159.pub3
Polyclonal anti-thymocyte globulins for the prophylaxis of graft-versus-host disease after allogeneic stem cell or bone marrow transplantation in adults
Abstract
Background: Allogeneic haematopoietic stem cell transplantation (SCT) is an established treatment for many malignant and non-malignant haematological disorders. Graft-versus-host disease (GVHD), a condition frequently occurring after an allogeneic SCT, is the result of host tissues being attacked by donor immune cells. It affects more than half of the patients after transplant either as acute and or chronic GVHD. One strategy for the prevention of GVHD is the administration of anti-thymocyte globulins (ATGs), a set of polyclonal antibodies directed against a variety of immune cell epitopes, leading to immunosuppression and immunomodulation.
Objectives: To assess the effect of ATG used for the prevention of GVHD in patients undergoing allogeneic SCT with regard to overall survival, incidence and severity of acute and chronic GVHD, incidence of relapse, non-relapse mortality, graft failure and adverse events.
Search methods: For this update we searched the CENTRAL, MEDLINE, Embase, trial registers and conference proceedings on the 18th November 2022 along with reference checking and contacting study authors to identify additional studies. We did not apply language restrictions.
Selection criteria: We included randomised controlled trials (RCTs) investigating the impact of ATG on GVHD prophylaxis in adults suffering from haematological diseases and undergoing allogeneic SCT. The selection criteria were modified from the previous version of this review. Paediatric studies and studies where patients aged < 18 years constituted more than 20 % of the total number were excluded. Treatment arms had to differ only in the addition of ATG to the standard GVHD prophylaxis regimen.
Data collection and analysis: We used standard methodological procedures expected by the Cochrane Collaboration for data collection, extraction and analyses.
Main results: For this update we included seven new RCTs, leading to a total of ten studies investigating 1413 participants. All patients had a haematological condition which warranted an allogeneic SCT. The risk of bias was estimated as low for seven and unclear for three studies. ATG probably has little or no influence on overall survival (HR (hazard ratio) 0.93 (95 % confidence interval (CI) 0.77 to 1.13, nine studies, n = 1249, moderate-certainty evidence)). Estimated absolute effect: 430 surviving people per 1000 people not receiving ATG compared to 456 people surviving per 1000 people receiving the intervention (95 % CI 385 to 522 per 1000 people). ATG results in a reduction in acute GVHD II to IV with relative risk (RR) 0.68 (95 % CI 0.60 to 0.79, 10 studies, n = 1413, high-certainty evidence). Estimated absolute effect: 418 acute GVHD II to IV per 1000 people not receiving ATG compared to 285 per 1000 people receiving the intervention (95 % CI 251 to 331 per 1000 people). Addition of ATG results in a reduction of overall chronic GvHD with a RR of 0.53 (95 % CI 0.45 to 0.61, eight studies, n = 1273, high-certainty evidence). Estimated absolute effect: 506 chronic GVHD per 1000 people not receiving ATG compared to 268 per 1000 people receiving the intervention (95 % CI 228 to 369 per 1000 people). Further data on severe acute GVHD and extensive chronic GVHD are available in the manuscript. ATG probably slightly increases the incidence of relapse with a RR of 1.21 (95 % CI 0.99 to 1.49, eight studies, n =1315, moderate-certainty evidence). Non relapse mortality is probably slightly or not affected by ATG with an HR of 0.86 (95 % CI 0.67 to 1.11, nine studies, n=1370, moderate-certainty evidence). ATG prophylaxis may result in no increase in graft failure with a RR of 1.55 (95 % CI 0.54 to 4.44, eight studies, n = 1240, low-certainty evidence). Adverse events could not be analysed due to the serious heterogeneity in the reporting between the studies, which limited comparability (moderate-certainty evidence) and are reported in a descriptive manner. Subgroup analyses on ATG types, doses and donor type are available in the manuscript.
Authors' conclusions: This systematic review suggests that the addition of ATG during allogeneic SCT probably has little or no influence on overall survival. ATG results in a reduction in the incidence and severity of acute and chronic GvHD. ATG intervention probably slightly increases the incidence of relapse and probably does not affect the non relapse mortality. Graft failure may not be affected by ATG prophylaxis. Analysis of data on adverse events was reported in a narrative manner. A limitation for the analysis was the imprecision in reporting between the studies thereby reducing the confidence in the certainty of evidence.
Trial registration: ClinicalTrials.gov NCT01856803 NCT02677181 NCT00655343 NCT00678275 NCT01295710 NCT01217723 NCT01883180.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
GC: No conflicts of interests
PF: No conflicts of interests
NS: No conflicts of interests; she is a co‐ordinating editor of Cochrane Haematology, but was not involved in the editorial process for this review.
HA:No conflicts of interests
ST: No conflicts of interests
Figures

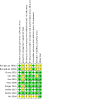

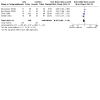

































Update of
-
Polyclonal anti-thymocyte globulins for the prophylaxis of graft-versus-host disease after allogeneic stem cell or bone marrow transplantation in adults.Cochrane Database Syst Rev. 2012 Sep 12;(9):CD009159. doi: 10.1002/14651858.CD009159.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2023 Jun 21;6:CD009159. doi: 10.1002/14651858.CD009159.pub3. PMID: 22972135 Updated.
References
References to studies included in this review
Bacigalupo 2001a {published data only}
-
- Bacigalupo A, Lamparelli T, Barisione G, Bruzzi P, Guidi S, Alessandrino PE et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biology of Blood and Marrow Transplantation 2006;12(5):560-5. - PubMed
-
- Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, Di Bartolomeo P et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood 2001;98(10):2942-7. - PubMed
Bacigalupo 2001b {published data only}
-
- Bacigalupo A, Lamparelli T, Barisione G, Bruzzi P, Guidi S, Alessandrino PE, et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biology of Blood and Marrow Transplantation 2006;12(5):560-5. - PubMed
-
- Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, Di Bartolomeo P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood 2001;98(10):2942-7. - PubMed
Chang 2020 {published data only}
-
- Chang YJ, Wu DP, Lai YR, Liu QF, Sun YQ, Hu J, et al. Antithymocyte globulin for matched sibling donor transplantation in patients with hematologic malignancies: A multicenter, open-label, randomized controlled study. Journal of Clinical Oncology 2020;38(29):JCO2000150. [DOI: 10.1200/JCO.20.00150] [PMID: ] - DOI - PubMed
Cho 2021 {published data only}
-
- Cho BY, Min GJ , Park SS, Yoon Yoon S, Park S, Jeon YW, et al. Low-dose thymoglobulin for prevention of chronic graft-versus-host disease in transplantation from an HLA-matched sibling donor. American Journal of Hematology 2021 Nov 1;96(11):1441-9. [PMID: ] - PubMed
-
- Min GJ, Cho BS, Park S‐S, Park S, Yahng S‐A, Jeon Y‐W, et al. A phase 3 trial of thymoglobuline for prevention of chronic GVHD intransplantation from an HLA-matched sibling. In: Blood. Vol. 136. 31.May.2021:32. [DOI: ]
Dou 2021 {published data only}
Finke 2009 {published data only}EUCTR2004‐000232‐91‐FI
-
- Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncology 2009;10(9):855-64. - PubMed
-
- Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Supplement to: Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised,open-label, multicentre phase 3 trial. Lancet Oncology published online August 19,2009. [DOI: ] - PubMed
-
- Finke J, Schmoor C, Bethge WA, Ottinger H, Stelljes M, Volin L, et al. Long-term outcomes after standard graft-versus-host disease prophylaxis with or without anti-human-T-lymphocyte immunoglobulin in haemopoietic cell transplantation from matched unrelated donors: final results of a randomised controlled trial. Lancet Haematology 2017;4(6):e293-e301. - PubMed
-
- Finke J, Schmoor C, Bethge WA, Ottinger H, Stelljes M, Volin L, et al. Randomized trial on GVHD prophylaxis with or without anti-human t-lymphocyte immunoglobulin ATG-fresenius (ATG-F) in allogeneic hematopoietic cell transplantation from matched unrelated donors: Final long-term results after 8.6 years median follow-up. In: Blood. Vol. 126. 2015:853.
-
- Finke J, Schmoor C, Bethge WA, Ottinger HD, Stelljes M, Zander AR, et al. Prognostic factors affecting outcome after allogeneic transplantation for hematological malignancies from unrelated donors: results from a randomized trial. Biology of Blood & Marrow Transplantation 2012;18(11):1716-26. - PubMed
Kroger 2016 {published data only}
-
- Bonifazi F, Solano C, Wolschke C, Sessa M, Patriarca F, Zallio F, et al. Acute GVHD prophylaxis plus ATLG after myeloablative allogeneic haemopoietic peripheral blood stem-cell transplantation from HLA-identical siblings in patients with acute myeloid leukaemia in remission: final results of quality of life and long-term outcome analysis of a phase 3 randomised study. Lancet Haematology 2019;6(2):e89-e99. - PubMed
-
- Bonifazi F, Solano C, Wolschke C, Sessa M, Pini M, Selleri C, et al. Significant Improvement of QoL by using ATG as part of the conditioning regimen followed by HLAidentical peripheral stem cell transplantation in acute leukemia patients. Results from a prospective, randomized phase III study (ATG Family Study). In: Bone Marrow Transplantation. Vol. 52. 2017:539-40.
-
- Bonifazi F, Solano C, Wolschke C, Sessa M, Zallio F, Selleri C, et al. Prophylaxis with ATLG significantly improves chronic graft-versus-host disease / relapse-free survival and quality of life after a myeloablative hla identical sibling transplant for acute leukemia patients in complete remission. Long-term follow-up of the randomized atg familystudy. In: Blood. Vol. 130. 2017.
-
- Kroger N, Solano C, Wolschke C, Bandini G, Patriarca F, Pini M, et al. Antilymphocyte globulin for prevention of chronic graft-versus-host disease. New England Journal of Medicine 2016;374(1):43-53. - PubMed
-
- Solano C, Bonifazi F, Wolschke C, Patriarca F, Pini M, Nagler A, et al. Improved cGvHD/relapse-free survival after HLA-identical sibling PBSC transplantation with ATG. A prospective multicenter randomized EBMT-labelled phase III trial (ATGfamilystudy). Bone Marrow Transplantation 2015;50:S1.
Soiffer 2017 {published data only}
-
- Soiffer RJ, Kim HT, Chen YB, Rybka W, Artz AS, Boyer M, et al. Impact of anti-T lymphocyte globulin (ATLG) on immune reconstitution in patients undergoing HLA matched unrelated myeloablative hematopoietic cell transplantation (HCT): Results from a prospective randomized double blind phase 3 clinical trial. In: Biology of Blood and Marrow Transplantation. Vol. 23. 2017:S170-S171.
-
- Soiffer RJ, Kim HT, McGuirk J, Horwitz ME, Johnston L, Patnaik MM, et al. A prospective randomized double blind phase 3 clinical trial of anti-T lymphocyte globulin (ATLG) to assess impact on chronic graft-versus-host disease (cGVHD) free survival in patients undergoing HLA matched unrelated myeloablative hematopoietic cell transplantation (HCT). In: Blood. Vol. 128. 2016. - PMC - PubMed
-
- Soiffer RJ, Kim HT, McGuirk J, Horwitz ME, Johnston L, Patnaik MM, et al. Prospective, randomized, double-blind, phase III clinical trial of anti-T-lymphocyte globulin to assess impact on chronic graft-versus-host disease-free survival in patients undergoing HLA-matched unrelated myeloablative hematopoietic cell transplantation. Journal of Clinical Oncology 2017;35(36):4003-11. - PMC - PubMed
Walker 2016 {published data only}
-
- Roy J, Panzarella T, Couban S, Couture F, Devins GM, Elemary M, et al. Less chronic graft-versus-host disease, immunosuppressive therapy and better survival after anti-thymocyte globulin in unrelated donor stem cell transplant recipients: longer follow-up of a multicentre cell therapy transplant Canada randomized trial. In: Blood. Vol. 134 (Supplement 1). 2019:875. [DOI: ]
-
- Walker I, Panzarella T, Couban S, Couture F, Devins G, Elemary M, et al. Addition of anti-thymocyte globulin to standard graft-versus-host disease prophylaxis versus standard treatment alone in patients with haematological malignancies undergoing transplantation from unrelated donors: final analysis of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematology 2020;7(2):e100‐e111. - PubMed
-
- Walker I, Panzarella T, Couban S, Couture F, Devins G, Elemary M, et al. Anti-thymocyte globulin (ATG) in patients with haematological malignancies undergoing haematopoietic cell transplantation (HCT) from unrelated donors: follow up of a CBMTG randomized trial. In: Bone Marrow Transplantation. Vol. 53. 2019:395‐6. [DOI: ]
-
- Walker I, Panzarella T, Couban S, Couture F, Devins G, Elemary M, et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: A randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncology 2016;17(2):164-713. - PubMed
-
- Walker I, Schultz KR, Toze CL, Kerr HM, Moore J, Szwajcer D, et al. Thymoglobulin decreases the need for immunosuppression at 12 months after myeloablative and nonmyeloablative unrelated donor transplantation: CBMTG 0801, a randomized, controlled trial. In: Blood. Vol. 124. 2014.
Wu 2004 {published data only}
-
- Wu BY, Guo KY, Song CY, Zhang AL, Yan DA, Yang YL, et al. Decrease of chronic graft-versus-host disease by adding anti-human thymocyte globulin to the conditioning regimen. Chung-Hua Hsueh Yeh Hsueh Tsa Chih: Chinese Journal of Hematology 2004;25(2):91-4. - PubMed
References to studies excluded from this review
Algarotti 2013 {published data only}
-
- Algarotti A, Mico C, Corradini P, Falda M, Alessandrino EP, Fanin R, et al. In vivo T cell depletion with antithymocyte globulin or alemtuzumab for unrelated donor stem cell transplantation with reduced intensity conditioning: Results of a multicenter randomized phase II clinical trial (the global study) from the gruppo italiano trapianto di midollo osseo (gitmo). In: Haematologica. Vol. 98. 2013:17.
-
- Algarotti A, Mico C, Corradini P, Falda M, Alessandrino EP, Fanin R, et al. In vivo T-cell depletion with antithymocyte globulin or alemtuzumab for unrelated donor stem cell transplantation with reduced intensity conditioning: Results of a multicentre randomized phase II clinical trial (the Global study) from the GITMO. In: Bone Marrow Transplantation. Vol. 48. 2013:S60-S61.
Algeri 2019 {published data only}
-
- Algeri M, Galimberti S, Bernardo ME, Rovelli A, Zecca M, Nasa GL, et al. Results of a multicentre, randomized, controlled open-label study on the use of anti-t-lymphocyte globulin (ATLG) and rituximab for immunomodulation of graft-versus-host disease (GvHD) and graft failure (GF) in patients with non-malignant disorders. In: Blood. Vol. 134. 2019.
Atta 2012 {published data only}
-
- Atta EH, Sousa AM, Schirmer MR, Bouzas LF, Nucci M, Abdelhay E. Different outcomes between cyclophosphamide plus horse or rabbit antithymocyte globulin for HLA-identical sibling bone marrow transplant in severe aplastic anemia. Biology of Blood & Marrow Transplantation 2012;18(12):1876-82. - PubMed
Bacigalupo 2002 {published data only}
-
- Bacigalupo A, Lamparelli T, Gualandi F, Bregante S, Raiola AM, Di Grazia C et al. Prophylactic antithymocyte globulin reduces the risk of chronic graft-versus-host disease in alternative-donor bone marrow transplants. Biology of Blood and Marrow Transplantation 2002;8(12):656-61. - PubMed
Bacigalupo 2010 {published data only}
-
- Bacigalupo A, Lamparelli T, Milone G, Sormani MP, Ciceri F, Peccatori J, et al. Pre-emptive treatment of acute GVHD: a randomized multicenter trial of rabbit anti-thymocyte globulin, given on day +7 after alternative donor transplants. Bone Marrow Transplantation 2010;45(2):385-91. - PubMed
Baron 2015 {published data only}
-
- Baron F, Zachee P, Maertens J, Kerre T, Ory A, Seidel L, et al. Non-myeloablative allogeneic hematopoietic cell transplantation following fludarabine plus 2 Gy TBI or ATG plus 8 Gy TLI: a phase II randomized study from the Belgian Hematological Society. Journal of Hematology & Oncology 2015;8:4. - PMC - PubMed
-
- Hannon M, Beguin Y, Ehx G, Servais S, Seidel L, Graux C, et al. Immune recovery after allogeneic hematopoietic stem cell transplantation following Flu-TBI versus TLI-ATG conditioning. Clinical Cancer Research 2015;21(14):3131-9. - PubMed
Bashir 2014 {published data only}
Champlin 2007 {published data only}
Chang 2018 {published data only}
-
- Chang Y, Wang Y, Mo XD, Zhang XH, Xu LP, Yan CH, et al. Optimal dose of antithymocyte globulin in conditioning regimens for unmanipulated haploidentical haematopoietic stem cell transplantation: Long-term outcomes of a prospective randomised trial. In: Blood. Vol. 128. 2016. - PubMed
-
- Chang YJ, Wang Y, Mo XD, Zhang XH, Xu LP, Yan CH, et al. Optimal dose of rabbit thymoglobulin in conditioning regimens for unmanipulated, haploidentical, hematopoietic stem cell transplantation: Long-term outcomes of a prospective randomized trial.[Erratum appears in Cancer. 2018 Feb 15;124(4):868; PMID: 29406585]. Cancer 2018;123(15):2881-92. - PubMed
Doney 1981 {published data only}
-
- Doney KC, Weiden PL, Storb R, Thomas ED. Failure of early administration of antithymocyte globulin to lessen graft-versus-host disease in human allogeneic marrow transplant recipients. Transplantation 1981;31(2):141-3. - PubMed
Lin 2019 {published data only}
-
- Lin R, Wang Y, Huang F, Fan Z, Zhang S, Yang T, et al. Influence of lower dose antithymocyteglobulin as conditioning regimen on viral infection after haploidentical allogeneic stem cell transplantation. In: Blood. Vol. 128. 2016.
-
- Lin R, Wang Y, Huang F, Fan Z, Zhang S, Yang T, et al. Two doses of antithymocyteglobulin as graft-versus-host disease prophylaxis in haploidentical allogeneic stem cell transplantation:aa multicenter prospective study. In: Blood. Vol. 128. 2016.
-
- Lin R, Wang Y, Yang T, Xu Y, Huang F, Fan Z, et al. EBV and CMV infection in recipients of haploidentical allogeneic stem cell transplantation receiving two different doses of antithymocyteglobulin as conditioning regimen. In: Haematologica. Vol. 101. 2016:629.
Mediavilla 2015 {published data only}
-
- Mediavilla C, Vigouroux S, Tabrizi R, Pigneux A, Duclos C, Mohr C, et al. Transient grades 3 to 4 acute hepatitis is a common complication of rabbit antithymocyte globulin (thymoglobulin) administered before allogeneic stem cell transplantation. Biology of Blood & Marrow Transplantation 2015;21(4):661-5. - PubMed
Mensen 2014 {published data only}
-
- Mensen A, Na IK, Hafer R, Meerbach A, Schlecht M, Pietschmann ML, et al. Comparison of different rabbit ATG preparation effects on early lymphocyte subset recovery after allogeneic HSCT and its association with EBV-mediated PTLD. Journal of Cancer Research & Clinical Oncology 2014;140(11):1971-80. - PMC - PubMed
Moiseev 2016 {published data only}
-
- Moiseev IS, Morozova EV, Rudnizkaya YV, Vlasova YU, Darskaya EI, Slesarchuk OA, et al. Update on the randomized trial of post-transplanation cyclophosphamide and rabbit ATG for graft-versus-host disease prophylaxis in chronic myeloproliferative neoplasms and myelodysplastic syndrome. Cellular Therapy and Transplantation 2016;5(3):49‐50.
Pidala 2011 {published data only}
Ramsay 1982 {published data only}
-
- Ramsay NK, Kersey JH, Robison LL, McGlave PB, Woods WG, Krivit W, et al. A randomized study of the prevention of acute graft-versus-host disease. New England Journal of Medicine 1982;306(7):392-7. - PubMed
Robin 2018 {published data only}
-
- Robin M, Raj K, Chevret S, Gauthier J, Lavallade H, Michonneau D, et al. Alemtuzumab versus anti-thymocyte globulin in patients transplanted from an unrelated donor after a reduced intensity conditioning. In: European Journal of Haematology. Vol. 30. 2018:30. - PubMed
Wang 2014 {published data only}
-
- Wang Y, Fu HX, Liu DH, Xu LP, Zhang XH, Chang YJ, et al. Influence of two different doses of antithymocyte globulin in patients with standard-risk disease following haploidentical transplantation: a randomized trial. Bone Marrow Transplantation 2014;49(3):426-33. - PubMed
-
- Wang Y, Huang x. Influence of two different doses of antithymocyte globulin on clinical outcomes of leukemia patients following haploidentical hematopoietic stem cell transplantation: results of a randomized trial. In: Haematologica. Vol. 98. 2013:230.
Weiden 1979 {published data only}
-
- Weiden PL, Doney K, Storb R, Thomas ED. Antihuman thymocyte globulin for prophylaxis of graft-versus-host disease. A randomized trial in patients with leukemia treated with HLA-identical sibling marrow grafts. Transplantation 1979;27(4):227-30. - PubMed
-
- Weiden PL, Doney K, Storb R, Thomas ED. Anti-human thymocyte globulin (ATG) for prophylaxis and treatment of graft-versus-host disease in recipients of allogeneic marrow grafts. Transplantation Proceedings 1978;10(1):213-6. - PubMed
References to ongoing studies
JPRN‐jRCTs031180435 {published data only}
-
- JPRN-jRCTs031180435. A randomized trial of ATG in HLA 1-locus mismatched unrelated bone marrow transplantation. https://rctportal.niph.go.jp/en/detail?trial_id=jRCTs031180435 2019.
JPRN‐UMIN000028008 {unpublished data only}
-
- JPRN-UMIN000028008. A randomized trial comparing conventional GVHD prophylaxis vs. anti-thymocyte globulin-combined GVHD prophylaxis in HLA 1-locus mismatched unrelated bone marrow transplantation. https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R00003... 2017.
NCT04203108 {unpublished data only}
-
- NCT04203108. ATG in HLA-matched sibling HSCT as GVHD prophylaxis. https://clinicaltrials.gov/show/NCT04203108 2019.
Additional references
Admiraal 2020
-
- Admiraal R, Boelens JJ. Anti-thymocyte globulin for GVHD: one dose does not fit all. Lancet Haematology 2020;7(7):e505. - PubMed
Arai 2015
-
- Arai S , Arora M, Wang T , Spellman SR , He W, Couriel DR, et al. Graft-versus-Host Disease Working Committee of the CIBMTR.Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the Center for International Blood and Marrow Transplant Research. Biology of Blood and Marrow Transplant 2015 Feb;21:266-74. [DOI: 10.1016/j.bbmt.2014.10.021] - DOI - PMC - PubMed
Arai 2017
-
- Arai Y, Jo T, Matsui H, Kondo T, Takaori-Kondo A. Efficacy of antithymocyte globulin for allogeneic hematopoietic cell transplantation: a systematic review and meta-analysis. Leukaemia and Lymphoma 2017;58(8):1840-8. - PubMed
Bachier 2021
-
- Bachier CR, Aggarwal SK, Hennegan K, Milgroom A, Francis K, Dehipawala S, et al. Epidemiology and treatment of chronic graft-versus-host disease post-allogeneic hematopoietic cell transplantation: A US Claims Analysis. Transplantation and Cell Therapy 2021 Jun;27:504.e1-504.e6. [DOI: 10.1016/j.jtct.2020.12.027] - DOI - PubMed
Bacigalupo 2005
Bacigalupo 2006
-
- Bacigalupo A, Lamparelli T, Barisione G, Bruzzi P, Guidi S, Alessandrino PE, et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biology of Blood and Marrow Transplantation 2006;12(5):560-5. - PubMed
Bacigalupo 2009
Barrett 2010
Braun 2021
CIBMTR US summary slides
-
- Auletta JJ, Kou J, Chen M, Shaw BE. Current use and outcome of hematopoietic stem cell transplantation. CIBMTR US summary slides 2021.
Ferrara 2009
Flowers 2011
Flowers 2015
Gagelmann 2017
-
- Gagelmann N, Ayuk F, Wolschke C, Kroger N. Comparison of different rabbit anti-thymocyte globulin formulations in allogeneic stem cell transplantation: systematic literature review and network meta-analysis. Biology of Blood & Marrow Transplantation 2017;23(12):2184-91. - PubMed
Ghimire 2017
Glucksberg 1974
-
- Glucksberg H, Storb R, Fefer A, Buckner C, Neiman P, Clift R. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974 October;18(4):295-304. - PubMed
GRADEpro GDT [Computer program]
-
- GRADEpro Guideline Development Tool. McMaster University, 2020, developed by Evidence Prime, Inc., 2020. Available from gradepro.org..
Greinix 2018
-
- Greinix HT, Eikema DJ, Koster L, Penack O, Yakoub-Agha I, Montoto S, et al. Incidence of acute graft-versus-host disease and survival after allogeneic hematopoietic cell transplantation over time: a study from the transplant complications and chronic malignancies working party of the EBMT. In: Blood. Vol. 132. 2018 Nov:Supplement 1.
Grüllich 2009
-
- Grüllich C, Ziegler C, Finke J. Rabbit anti T-lymphocyte globulin induces apoptosis in peripheral blood mononuclear cell compartments and leukemia cells, while hematopoetic stem cells are apoptosis resistant. Biology of Blood and Marrow Transplantation 2009 February;15(2):173-82. - PubMed
Gyurkocza 2014
Higgins 2011
Higgins 2022
-
- Higgins JPT Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Vol. Available from www.training.cochrane.org/handbook. Cochrane, 2022.
Horwitz 2006
Jagasia 2015
-
- Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biology of Blood and Marrow Transplantation 2015;21(3):389-401.e1. - PMC - PubMed
Kumar 2019
-
- Kumar A, Reljic T, Hamadani M, Mohty M, Kharfan-Dabaja MA. Antithymocyte globulin for graft-versus-host disease prophylaxis: an updated systematic review and meta-analysis. Bone Marrow Transplantation 2019;54(7):1094-106. - PubMed
Lee 2013
-
- Lee SE, Cho BS, Kim JH, Yoon JH, Shin SH, Yahng SA, et al. Risk and prognostic factors for acute GVHD based on NIH consensus criteria. Bone Marrow Transplantation 2013;48(4):587-92. - PubMed
Lee 2017
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editors(s). Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org. The Cochrane Collaboration, 2011.
Lopez 2006
-
- Lopez M, Clarkson MR, Albin M, Sayegh MH, Najafian N. A novel mechanism of action for anti-thymocyte globulin: induction of CD4+CD25+Foxp3+ regulatory T cells. Journal of the American Society of Nephrology 2006 October;17(10):2844-53. - PubMed
Martinez‐Cibrian 2021
-
- Martinez-Cibrian N, Zeiser R, Perez-Simon JA. Graft-versus-host disease prophylaxis: Pathophysiology-based review on current approaches and future directions. Blood Reviews 2021 July;48:Epub. [EMBASE: 10.1016/j.blre.2020.100792] - PubMed
Moher 2009
-
- Moher D, Liberati A, Tetzlaff J, Altman DG, The Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. - PubMed
Moreno 2019
-
- Moreno DF, Cid J. Graft-versus-host disease. Medicina Clinica 2019;152(1):22-8. - PubMed
Mothy 2007
Niederwieser 2022
-
- Niederwieser D, Baldomero H, Bazuaye N, Bupp C, Chaudhri N, Corbacioglu S, et al. One and a half million hematopoietic stem cell transplants: continuous and differential improvement in worldwide access with the use of non-identical family donors. Haematologica 2022 May 1;1:1045-53. [DOI: 10.3324/haematol.2021.279189] [PMID: ] - DOI - PMC - PubMed
Olsson 2015
Paczesny 2010
Page 2021
-
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [PMID: ] - PubMed
Passweg 2020
Penack 2020
-
- Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation.. Lancet Haematology 2020 Feb;7:e157-e167. [DOI: 10.1016/S2352-3026(19)30256-X] - DOI - PubMed
Ramachandran 2019
-
- Ramachandran V, Kolli SS, Strowd LC. Review of graft-versus-host disease. Dermatologic Clinics 2019;37(4):569-82. - PubMed
RevMan Web 2021 [Computer program]
-
- Review Manager Web (RevMan Web). Version Version: 2.5.0. The Cochrane Collaboration, 2021, 06 Apr. available at revman.cochrane.org.
Santesso 2020
-
- Santesso N, Glenton C, Dahm P, Garner P, Akl A, Alper B, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020;119:126-35. - PubMed
Siddiqui 2019
Skoetz 2020
-
- Skoetz N, Goldkuhle M, Van Dalen EC, Akl EA, Trivella M, Mustafa RA, et al. GRADE guidelines 27: how to calculate absolute effects for time-to-event outcomes in summary of findings tables and evidence profiles. Journal of Clinical Epidemiology 2020;118:124-31. - PubMed
Socié 2011
-
- Socié G, Schmoor C, Bethge WA, Ottinger HD, Stelljes M, Zander AR, et al. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood 2011 June;117(23):6375-82. - PubMed
Tierney 2007
TIjon 2021
Valcaercel 2019
-
- Valcaercel D, Sureda A. Graft failure. In: EBMT Handbook 2019. Springer, 2019. [DOI: ] - PubMed
Wingard 2011
Zeiser 2020
References to other published versions of this review
Theurich 2011
-
- Theurich S, Fischmann H, Shimabukuro‐Vornhagen A, Skoetz N, Chemnitz JM, Holtick U, et al. Polyclonal anti‐thymocyte globulins for the prophylaxis of graft‐versus‐host disease after allogeneic stem cell/bone marrow transplantation. Cochrane Database of Systematic Reviews 2011, Issue 6. Art. No: CD009159. [DOI: 10.1002/14651858.CD009159] - DOI - PubMed
Theurich 2012
-
- Theurich S, Fischmann H, Shimabukuro‐Vornhagen A, Chemnitz JM, Holtick U, Scheid C, et al. Polyclonal anti‐thymocyte globulins for the prophylaxis of graft‐versus‐host disease after allogeneic stem cell or bone marrow transplantation in adults. Cochrane Database of Systematic Reviews 2012, Issue 9. Art. No: CD009159. [DOI: 10.1002/14651858.CD009159.pub2] - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

