Pulmonary microbiota intervention alleviates fine particulate matter-induced lung inflammation in broilers
- PMID: 37341706
- PMCID: PMC10390102
- DOI: 10.1093/jas/skad207
Pulmonary microbiota intervention alleviates fine particulate matter-induced lung inflammation in broilers
Abstract
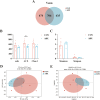
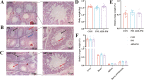
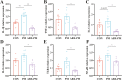
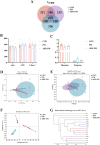
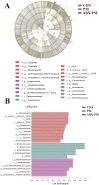
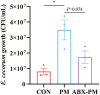
Fine particulate matter (PM2.5) released during the livestock industry endangers the respiratory health of animals. Our previous findings suggested that broilers exposed to PM2.5 exhibited lung inflammation and changes in the pulmonary microbiome. Therefore, this study was to investigate whether the pulmonary microbiota plays a causal role in the pathogenesis of PM2.5-induced lung inflammation. We first used antibiotics to establish a pulmonary microbiota intervention broiler model, which showed a significantly reduced total bacterial load in the lungs without affecting the microbiota composition or structure. Based on it, 45 AA broilers of similar body weight were randomly assigned to three groups: control (CON), PM2.5 (PM), and pulmonary microbiota intervention (ABX-PM). From 21 d of age, broilers in the ABX-PM group were intratracheally instilled with antibiotics once a day for 3 d. Meanwhile, broilers in the other two groups were simultaneously instilled with sterile saline. On 24 and 26 d of age, broilers in the PM and ABX-PM groups were intratracheally instilled with PM2.5 suspension to induce lung inflammation, and broilers in the CON group were simultaneously instilled with sterile saline. The lung histomorphology, inflammatory cytokines' expression levels, lung microbiome, and microbial growth conditions were analyzed to determine the effect of the pulmonary microbiota on PM2.5-induced lung inflammation. Broilers in the PM group showed lung histological injury, while broilers in the ABX-PM group had normal lung histomorphology. Furthermore, microbiota intervention significantly reduced mRNA expression levels of interleukin-1β, tumor necrosis factor-α, interleukin-6, interleukin-8, toll-like receptor 4 and nuclear factor kappa-B. PM2.5 induced significant changes in the β diversity and structure of the pulmonary microbiota in the PM group. However, no significant changes in microbiota structure were observed in the ABX-PM group. Moreover, the relative abundance of Enterococcus cecorum in the PM group was significantly higher than that in the CON and ABX-PM groups. And sterile bronchoalveolar lavage fluid from the PM group significantly promoted the growth of E. cecorum, indicating that PM2.5 altered the microbiota's growth condition. In conclusion, pulmonary microbiota can affect PM2.5-induced lung inflammation in broilers. PM2.5 can alter the bacterial growth environment and promote dysbiosis, potentially exacerbating inflammation.
Keywords: broiler house; fine particulate matter; lung inflammation; microbiome; pulmonary microbiota.
Plain language summary
Fine particulate matter (PM2.5) in broiler houses has a negative impact on broiler respiratory tracts, and PM2.5 exposure can induce lung inflammation and cause microbiota dysbiosis. The pulmonary microbiota is involved in maintaining immune homeostasis in the lungs, and a variety of lung diseases exhibit microbiota disturbances. However, the correlation between the pulmonary microbiota and PM2.5-induced lung inflammation is poorly understood. This study aimed to investigate whether the pulmonary microbiota influenced PM2.5-induced lung inflammation. We use antibiotics to reduce the quantity of bacteria in the lungs without destroying their composition. PM2.5 was then used to induce lung inflammation in both untreated and intervened pulmonary microbiota broilers. Compared to untreated microbiota broilers, intervened microbiota broilers had less morphological lung tissue injury and lower inflammatory factor expression levels after PM2.5 exposure. Furthermore, the intervened microbiota broilers’ microbiota structure remained normal, while the untreated microbiota broilers showed dysbiosis. This dysbiosis is closely linked to changes in the microbial growth environment due to the inflammatory response. This suggested that the pulmonary microbiota affects PM2.5-induced lung inflammation in broilers. Dysbiosis caused by inflammation that alters the conditions for bacterial growth may exacerbate inflammation.
© The Author(s) 2023. Published by Oxford University Press on behalf of the American Society of Animal Science. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Barfod, K. K., Vrankx K., Mirsepasi-Lauridsen H. C., Hansen J. S., Hougaard K. S., Larsen S. T., Ouwenhand A. C., and Krogfelt K. A.. . 2015. The murine lung microbiome changes during lung inflammation and intranasal vancomycin treatment. Open Microbiol. J. 9:167–179. doi: 10.2174/1874285801509010167 - DOI - PMC - PubMed
-
- Budden, K. F., Shukla S. D., Rehman S. F., Bowerman K. L., Keely S., Hugenholtz P., Armstrong-James D. P. H., Adcock I. M., Chotirmall S. H., Chung K. F., . et al. 2019. Functional effects of the microbiota in chronic respiratory disease. Lancet. Respir. Med. 7:907–920. doi: 10.1016/S2213-2600(18)30510-1 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

