The spontaneous mouse mutant low set ears (Lse) is caused by tandem duplication of Fgf3 and Fgf4
- PMID: 37341808
- PMCID: PMC11601887
- DOI: 10.1007/s00335-023-09999-8
The spontaneous mouse mutant low set ears (Lse) is caused by tandem duplication of Fgf3 and Fgf4
Abstract
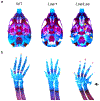
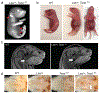
The external ear develops from an organized convergence of ventrally migrating neural crest cells into the first and second branchial arches. Defects in external ear position are often symptomatic of complex syndromes such as Apert, Treacher-Collins, and Crouzon Syndrome. The low set ears (Lse) spontaneous mouse mutant is characterized by the dominant inheritance of a ventrally shifted external ear position and an abnormal external auditory meatus (EAM). We identified the causative mutation as a 148 Kb tandem duplication on Chromosome 7, which includes the entire coding sequences of Fgf3 and Fgf4. Duplications of FGF3 and FGF4 occur in 11q duplication syndrome in humans and are associated with craniofacial anomalies, among other features. Intercrosses of Lse-affected mice revealed perinatal lethality in homozygotes, and Lse/Lse embryos display additional phenotypes including polydactyly, abnormal eye morphology, and cleft secondary palate. The duplication results in increased Fgf3 and Fgf4 expression in the branchial arches and additional discrete domains in the developing embryo. This ectopic overexpression resulted in functional FGF signaling, demonstrated by increased Spry2 and Etv5 expression in overlapping domains of the developing arches. Finally, a genetic interaction between Fgf3/4 overexpression and Twist1, a regulator of skull suture development, resulted in perinatal lethality, cleft palate, and polydactyly in compound heterozygotes. These data indicate a role for Fgf3 and Fgf4 in external ear and palate development and provide a novel mouse model for further interrogation of the biological consequences of human FGF3/4 duplication.
© 2023. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Competing Interests: The corresponding author, Stephen A. Murray, is an editor-in-chief of the journal. There are no other competing interests.
Figures




References
-
- Bourgeois P, Bolcato-Bellemin AL, Danse JM, Bloch-Zupan A, Yoshiba K, Stoetzel C, Perrin-Schmitt F, 1998. The variable expressivity and incomplete penetrance of the twist-null heterozygous mouse phenotype resemble those of human Saethre-Chotzen syndrome. Hum Mol Genet 7, 945–957. - PubMed
-
- Carlton MB, Colledge WH, Evans MJ, 1998. Crouzon-like craniofacial dysmorphology in the mouse is caused by an insertional mutation at the Fgf3/Fgf4 locus. Dev Dyn 212, 242–249. - PubMed
-
- Carver EA, Oram KF, Gridley T, 2002. Craniosynostosis in Twist heterozygous mice: a model for Saethre-Chotzen syndrome. Anat Rec 268, 90–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

