Tau seeding and spreading in vivo is supported by both AD-derived fibrillar and oligomeric tau
- PMID: 37341831
- PMCID: PMC10329061
- DOI: 10.1007/s00401-023-02600-1
Tau seeding and spreading in vivo is supported by both AD-derived fibrillar and oligomeric tau
Abstract
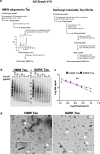
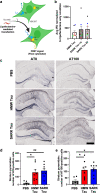
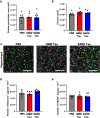
Insoluble fibrillar tau, the primary constituent of neurofibrillary tangles, has traditionally been thought to be the biologically active, toxic form of tau mediating neurodegeneration in Alzheimer's disease. More recent studies have implicated soluble oligomeric tau species, referred to as high molecular weight (HMW), due to their properties on size-exclusion chromatography, in tau propagation across neural systems. These two forms of tau have never been directly compared. We prepared sarkosyl-insoluble and HMW tau from the frontal cortex of Alzheimer patients and compared their properties using a variety of biophysical and bioactivity assays. Sarkosyl-insoluble fibrillar tau comprises abundant paired-helical filaments (PHF) as quantified by electron microscopy (EM) and is more resistant to proteinase K, compared to HMW tau, which is mostly in an oligomeric form. Sarkosyl-insoluble and HMW tau are nearly equivalent in potency in HEK cell bioactivity assay for seeding aggregates, and their injection reveals similar local uptake into hippocampal neurons in PS19 Tau transgenic mice. However, the HMW preparation appears to be far more potent in inducing a glial response including Clec7a-positive rod microglia in the absence of neurodegeneration or synapse loss and promotes more rapid propagation of misfolded tau to distal, anatomically connected regions, such as entorhinal and perirhinal cortices. These data suggest that soluble HMW tau has similar properties to fibrillar sarkosyl-insoluble tau with regard to tau seeding potential, but may be equal or even more bioactive with respect to propagation across neural systems and activation of glial responses, both relevant to tau-related Alzheimer phenotypes.
Keywords: Alzheimer; Microglia; Seeding; Spreading; Tau.
© 2023. The Author(s).
Figures




Update of
-
Tau seeding and spreading in vivo is supported by both AD-derived fibrillar and oligomeric tau.bioRxiv [Preprint]. 2023 May 26:2023.03.28.534418. doi: 10.1101/2023.03.28.534418. bioRxiv. 2023. Update in: Acta Neuropathol. 2023 Aug;146(2):191-210. doi: 10.1007/s00401-023-02600-1. PMID: 37034629 Free PMC article. Updated. Preprint.
Comment in
-
Soluble oligomers or insoluble fibrils? Scientific commentary on "Tau seeding and spreading in vivo is supported by both AD-derived fibrillar and oligomeric tau".Acta Neuropathol. 2023 Dec;146(6):861-862. doi: 10.1007/s00401-023-02633-6. Epub 2023 Sep 21. Acta Neuropathol. 2023. PMID: 37733037 No abstract available.
References
-
- Alzheimer A. über eigenartige Krankheitsfälle des späteren Alters. Z Für Gesamte Neurol Psychiatr. 1911;4:356–385. doi: 10.1007/BF02866241. - DOI
-
- Audouard E, Houben S, Masaracchia C, Yilmaz Z, Suain V, Authelet M, et al. High-molecular-weight paired helical filaments from alzheimer brain induces seeding of wild-type mouse tau into an argyrophilic 4R tau pathology in vivo. Am J Pathol. 2016;186:2709–2722. doi: 10.1016/j.ajpath.2016.06.008. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

