Model-Informed Support of Dose Selection for Prophylactic Treatment with Dalcinonacog Alfa in Adult and Paediatric Hemophilia B Patients
- PMID: 37341915
- PMCID: PMC10427527
- DOI: 10.1007/s12325-023-02570-6
Model-Informed Support of Dose Selection for Prophylactic Treatment with Dalcinonacog Alfa in Adult and Paediatric Hemophilia B Patients
Abstract
Introduction: Dalcinonacog alfa (DalcA), a novel subcutaneously administered recombinant human factor IX (FIX) variant is being developed for adult and paediatric patients with hemophilia B (HB). DalcA has been shown to raise FIX to clinically meaningful levels in adults with HB. This work aimed to support dosing regimen selection in adults and perform first-in-paediatric dose extrapolations using a model-based pharmacokinetic (PK) approach.
Methods: A population PK model was built using adult data from two clinical trials (NCT03186677, NCT03995784). With allometry in the model, clinical trial simulations were performed to study alternative dosing regimens in adults and children. Steady-state trough levels and the time-to-reach target were derived to inform dose selection.
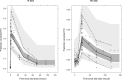
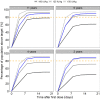
Results: Almost 90% of the adults were predicted to achieve desirable FIX levels, i.e. 10% FIX activity, following daily 100 IU/kg dosing, with 90% of the subjects reaching target within 1.6-7.1 days. No every-other-day regimen met the target. A dose of 125 IU/kg resulted in adequate FIX levels down to 6 years, whereas a 150 IU/kg dose was needed below 6 down to 2 years of age. For subjects down to 6 years that did not reach target with 125 IU/kg, a dose escalation to 150 IU/kg was appropriate. The children below 6 to 2 years were shown to need a dose escalation to 200 IU/kg if 150 IU/kg given daily was insufficient.
Conclusion: This study supported the adult dose selection for DalcA in the presence of sparse data and enabled first-in-paediatric dose selection to achieve FIX levels that reduce risk of spontaneous bleeds.
Keywords: Dalcinonacog alfa; Dose selection; Hemophilia B; MID3; Population pharmacokinetics.
© 2023. The Author(s).
Conflict of interest statement
Natacha Le Moan, Eduard Gorina, Grant E. Blouse and Tom Knudsen were employees and Natacha Le Moan and Tom Knudsen were shareholders of Catalyst Biosciences when this work was performed. Ulrika S.H. Simonsson received funding for this work. Alan Faraj have no conflict of interest to declare. Natacha Le Moan and Eduard Gorina changed affiliation prior to completion of the manuscript (Ashvattha Therapeutics, Inc and Freelance, respectively).
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

