Use of Ambroxol as Therapy for Gaucher Disease
- PMID: 37342037
- PMCID: PMC10285580
- DOI: 10.1001/jamanetworkopen.2023.19364
Use of Ambroxol as Therapy for Gaucher Disease
Abstract
Importance: Ambroxol was identified as an enhancer of stability and residual activity of several misfolded glucocerebrosidase variants in 2009.
Objectives: To assess hematologic and visceral outcomes, biomarker changes, and safety of ambroxol therapy for patients with Gaucher disease (GD) without disease-specific treatment.
Design, setting, and participants: Patients with GD who could not afford enzyme replacement therapy were enrolled and received oral ambroxol from May 6, 2015, to November 9, 2022, at Xinhua Hospital, affiliated with Shanghai Jiao Tong University School of Medicine, Shanghai, China. Thirty-two patients with GD (29 with GD type 1, 2 with GD type 3, and 1 with GD intermediate types 2-3) were enrolled. Of those, 28 patients were followed up for longer than 6 months; 4 were excluded due to loss of follow-up. Data analyses were performed from May 2015 to November 2022.
Intervention: An escalating dose of oral ambroxol (mean [SD] dose, 12.7 [3.9] mg/kg/d).
Main outcomes and measures: Patients with GD receiving ambroxol were followed up in a genetic metabolism center. Biomarkers of chitotriosidase activity and glucosylsphingosine level, liver and spleen volumes, and hematologic parameters were measured at baseline and various time points throughout the ambroxol treatment.
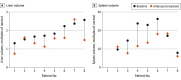
Results: A total of 28 patients (mean [SD] age, 16.9 [15.3] years; 15 male patients [53.6%]) received ambroxol for a mean (SD) duration of 2.6 (1.7) years. Two patients with severe symptoms at baseline experienced deterioration of hematologic parameters and biomarkers and were deemed nonresponders; clinical response was observed in the other 26 patients. After 2.6 years of ambroxol treatment, the mean (SD) hemoglobin concentration improved from 10.4 (1.7) to 11.9 (1.7) g/dL (mean [SD], 1.6 [1.7] g/dL; 95% CI, 0.8-2.3 g/dL; P < .001), and the mean (SD) platelet count improved from 69 (25) to 78 (30) × 103/µL (mean [SD], 9 [22] × 103/µL; 95% CI, -2 to 19 × 103/µL; P = .09). The mean (SD) spleen volume decreased from 17.47 (7.18) to 12.31 (4.71) multiples of normal (MN) (mean [SD], -5.16 [5.44] MN; 95% CI, -10.19 to -0.13; P = .04), and the mean (SD) liver volume decreased from 1.90 (0.44) to 1.50 (0.53) MN (mean [SD], -0.39 [0.42] MN; 95% CI, -0.75 to -0.04; P = .03). Biomarker median percentage changes from baseline were -43.1% for chitotriosidase activity (from 14 598 [range, 3849-29 628] to 8312 [range, 1831-16 842] nmol/mL/h; z = -3.413; P = .001) and -34.1% for glucosylsphingosine level (from 251.3 [range, 73.6-944.2] to 165.7 [range, 21.3-764.8] ng/mL; z = -2.756; P = .006). Patients were divided into subgroups according to age when initiating treatment; those who received treatment at a younger age (mean [SD] age, 6.3 [2.7] years) experienced more rapid improvements: hemoglobin concentration increased by 16.5% (from 10.3 [1.5] to 12.0 [1.5] g/dL; mean [SD] change, 1.6 [1.6] g/dL; 95% CI, 0.7-2.5 g/dL; P = .002), and platelet count increased by 12.0% (from 75 [24] to 84 [33] × 103/µL; mean [SD] change, 9 [26] × 103/µL; 95% CI, -5 to 24 × 103/µL; P = .17); whereas chitotriosidase activity decreased by 64.0% (from 15 710 [range, 4092-28 422] to 5658 [range, 1146-16 843] nmol/mL/h; z = -2.803; P = .005), and glucosylsphingosine level decreased by 47.3% (from 248.5 [range, 122.8-674.9] to 131.0 [range, 41.1-448.5] ng/mL; z = -2.385; P = .02). Three of the 28 patients experienced mild and transient adverse events.
Conclusions and relevance: In this case series of ambroxol repurposing among patients with GD, long-term treatment with ambroxol was safe and associated with patient improvement. Improvements in hematologic parameters, visceral volumes, and plasma biomarkers were larger among patients with relatively mild symptoms of GD and patients who received initial treatment at younger ages.
Conflict of interest statement
Figures


Comment in
-
Ambroxol as Therapy for Gaucher Disease-Ambitious but Ambivalent.JAMA Netw Open. 2023 Jun 1;6(6):e2319336. doi: 10.1001/jamanetworkopen.2023.19336. JAMA Netw Open. 2023. PMID: 37342045 No abstract available.
Similar articles
-
An open-label, noncomparative study of miglustat in type I Gaucher disease: efficacy and tolerability over 24 months of treatment.Clin Ther. 2005 Aug;27(8):1215-27. doi: 10.1016/j.clinthera.2005.08.004. Clin Ther. 2005. PMID: 16199246 Clinical Trial.
-
Velaglucerase alfa for the management of type 1 Gaucher disease.Clin Ther. 2012 Feb;34(2):259-71. doi: 10.1016/j.clinthera.2011.12.017. Epub 2012 Jan 20. Clin Ther. 2012. PMID: 22264444 Review.
-
Gaucher disease type 1 patients from the ICGG Gaucher Registry sustain initial clinical improvements during twenty years of imiglucerase treatment.Mol Genet Metab. 2021 Feb;132(2):100-111. doi: 10.1016/j.ymgme.2020.12.295. Epub 2021 Jan 8. Mol Genet Metab. 2021. PMID: 33485799
-
[Application of plasma glucosylsphingosine detection in the follow-up of patients with Gaucher disease].Zhonghua Yi Xue Za Zhi. 2020 Nov 3;100(40):3169-3173. doi: 10.3760/cma.j.cn112137-20200717-02138. Zhonghua Yi Xue Za Zhi. 2020. PMID: 33142401 Chinese.
-
Rapid and long-lasting efficacy of high-dose ambroxol therapy for neuronopathic Gaucher disease: A case report and literature review.Mol Genet Genomic Med. 2024 Apr;12(4):e2427. doi: 10.1002/mgg3.2427. Mol Genet Genomic Med. 2024. PMID: 38553911 Free PMC article. Review.
Cited by
-
Cost-effectiveness of ambroxol in the treatment of Gaucher disease type 2.Open Med (Wars). 2024 May 21;19(1):20240970. doi: 10.1515/med-2024-0970. eCollection 2024. Open Med (Wars). 2024. PMID: 38799251 Free PMC article.
-
Ambroxol as a Treatment for Parkinson Disease Dementia: A Randomized Clinical Trial.JAMA Neurol. 2025 Jun 30;82(8):797-807. doi: 10.1001/jamaneurol.2025.1687. Online ahead of print. JAMA Neurol. 2025. PMID: 40587145
-
GBA1-Associated Parkinson's Disease Is a Distinct Entity.Int J Mol Sci. 2024 Jun 28;25(13):7102. doi: 10.3390/ijms25137102. Int J Mol Sci. 2024. PMID: 39000225 Free PMC article. Review.
-
Exploring the efficacy and safety of Ambroxol in Gaucher disease: an overview of clinical studies.Front Pharmacol. 2024 Feb 13;15:1335058. doi: 10.3389/fphar.2024.1335058. eCollection 2024. Front Pharmacol. 2024. PMID: 38414738 Free PMC article. Review.
-
Functional Analysis of Human GBA1 Missense Mutations in Drosophila: Insights into Gaucher Disease Pathogenesis and Phenotypic Consequences.Cells. 2024 Sep 27;13(19):1619. doi: 10.3390/cells13191619. Cells. 2024. PMID: 39404383 Free PMC article.

