High-definition Cathodal Direct Current Stimulation for Treatment of Acute Ischemic Stroke: A Randomized Clinical Trial
- PMID: 37342040
- PMCID: PMC10285579
- DOI: 10.1001/jamanetworkopen.2023.19231
High-definition Cathodal Direct Current Stimulation for Treatment of Acute Ischemic Stroke: A Randomized Clinical Trial
Abstract
Importance: Cathodal transcranial direct current stimulation (C-tDCS) provides neuroprotection in preclinical models of acute ischemic stroke (AIS) by inhibiting peri-infarct excitotoxic effects and enhancing collateral perfusion due to its vasodilatory properties.
Objective: To report the first-in-human pilot study using individualized high-definition (HD) C-tDCS as a treatment of AIS.
Design, setting, and participants: This randomized clinical trial was sham controlled with 3 + 3 dose escalation design, and was conducted at a single center from October 2018 to July 2021. Eligible participants were treated for AIS within 24 hours from onset, had imaging evidence of cortical ischemia with salvageable penumbra, and were ineligible for reperfusion therapies. HD C-tDCS electrode montage was selected for each patient to deliver the electric current to the ischemic region only. Patients were followed for 90 days.
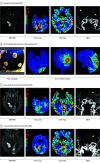
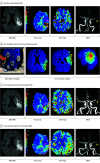
Main outcomes and measures: Primary outcomes were feasibility, assessed as time from randomization to study stimulation initiation; tolerability, assessed by rate of patients completing the full study stimulation period; and safety, assessed by rates of symptomatic intracranial hemorrhage at 24 hours. The efficacy imaging biomarkers of neuroprotection and collateral enhancement were explored.
Results: A total of 10 patients with AIS were enrolled, 7 were randomized to active treatment and 3 to sham. Patient age was mean (SD) 75 (10) years old, 6 (60%) were female, and National Institutes of Health Stroke Scale score was mean (SD) 8 (7). Two doses of HD C-tDCS (1 milliamp [mA] for 20 minutes and 2 mA for 20 minutes) were studied. The speed of HD C-tDCS implementation was a median (IQR) 12.5 minutes (9-15 minutes) in the last 4 patients. Patients tolerated the HD C-tDCS with no permanent stimulation cessation. The hypoperfused region was reduced by a median (IQR) 100% (46% to 100%) in the active group vs increased by 325% (112% to 412%) in sham. Change in quantitative relative cerebral blood volume early poststimulation was a median (IQR) 64% (40% to 110%) in active vs -4% (-7% to 1%) sham patients and followed a dose-response pattern. Penumbral salvage in the active C-tDCS group was median (IQR) 66% (29% to 80.5%) vs 0% (IQR 0% to 0%) in sham.
Conclusion and relevance: In this randomized, first-in-human clinical trial, HD C-tDCS was started efficiently and well tolerated in emergency settings, with signals of beneficial effect upon penumbral salvage. These results support advancing HD C-tDCS to larger trials.
Trial registration: ClinicalTrials.gov Identifier: NCT03574038.
Conflict of interest statement
Figures


References
-
- Virani SS, Alonso A, Aparicio HJ, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254-e743. doi: 10.1161/CIR.0000000000000950 - DOI - PubMed

