Mortierellaceae from subalpine and alpine habitats: new species of Entomortierella, Linnemannia, Mortierella, Podila and Tyroliella gen. nov
- PMID: 37342154
- PMCID: PMC10277274
- DOI: 10.3114/sim.2022.103.02
Mortierellaceae from subalpine and alpine habitats: new species of Entomortierella, Linnemannia, Mortierella, Podila and Tyroliella gen. nov
Abstract
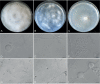
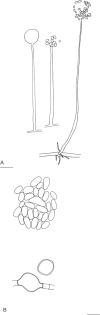
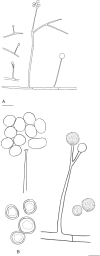
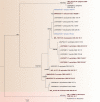
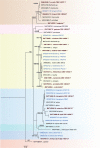
Fungi are incredibly diverse, but they are unexplored, especially in the subalpine and alpine zone. Mortierellaceae are certainly one of the most abundant, species-rich, and widely distributed cultivable soil fungal families in terrestrial habitats, including subalpine and alpine zones. The phylogeny of Mortierellaceae was recently resolved based on current state of the art molecular techniques, and the paraphyletic genus Mortierella sensu lato (s.l.) was divided into 13 monophyletic genera. Our extensive sampling campaigns in the Austrian Alps resulted in 139 different Mortierellaceae pure culture isolates representing 13 new species. For the definition of taxa, we applied both classical morphological criteria, as well as modern DNA-based methods. Phylogenetic relationships were resolved based on the ribosomal DNA internal transcribed spacer (rDNA ITS), the large subunit (LSU), and the DNA-directed RNA polymerase II largest subunit 1 (RPB1). In this study, we proposed a new genus and described 13 new species belonging to the genera Entomortierella, Linnemannia, Mortierella and Podila. In addition, we proposed eight new combinations, re-defined E. jenkinii at species level, defined a neotype for M. alpina and lecto- as well as epitypes for M. fatshederae, M. jenkinii, and M. longigemmata. The rDNA ITS region is generally applied as classical barcoding gene for fungi. However, the obtained phylogenetic resolution is often too low for an accurate identification of closely related species of Mortierellaceae, especially for small sampling sizes. In such cases, unambiguous identification can be obtained based on morphological characters of pure culture isolates. Therefore, we also provide dichotomous keys for species identification within phylogenetic lineages. Taxonomic novelties: new genus: Tyroliella Telagathoti, Probst & Peintner; New species: Entomortierella galaxiae Telagathoti, M. Probst & Peintner, Linnemannia bainierella Telagathoti, M. Probst & Peintner, Linnemannia stellaris Telagathoti, M. Probst & Peintner, Linnemannia nimbosa Telagathoti, M. Probst & Peintner, Linnemannia mannui Telagathoti, M. Probst & Peintner, Linnemannia friederikiana Telagathoti, M. Probst & Peintner, Linnemannia scordiella Telagathoti, M. Probst & Peintner, Linnemannia solitaria Telagathoti, M. Probst & Peintner, Mortierella triangularis Telagathoti, M. Probst & Peintner, Mortierella lapis Telagathoti, M. Probst & Peintner, Podila himami Telagathoti, M. Probst & Peintner, Podila occulta Telagathoti, M. Probst & Peintner, Tyroliella animus-liberi Telagathoti, Probst & Peintner; New combinations: Entomortierella basiparvispora (W. Gams & Grinb.) Telagathoti, M. Probst & Peintner, Entomortierella jenkinii (A.L. Sm.) Telagathoti, M. Probst & Peintner; Entomortierella sugadairana (Y. Takash. et al.) Telagathoti, M. Probst & Peintner, Linnemannia zonata (Linnem. ex W. Gams) Telagathoti, M. Probst & Peintner, Linnemannia fluviae (Hyang B. Lee et al.) Telagathoti, M. Probst & Peintner, Linnemannia biramosa (Tiegh.) Telagathoti, M. Probst & Peintner, Linnemannia cogitans (Degawa) Telagathoti, M. Probst & Peintner, Tyroliella pseudozygospora (W. Gams & Carreiro) Telagathoti, M. Probst & Peintner; Epitypifications (basionyms): Mortierella bainieri var. jenkinii A.L. Sm., Mortierella fatshederae Linnem., Mortierella longigemmata Linnem. Neotypification (basionym): Mortierella alpina Peyronel. Citation: Telagathoti A, Probst M, Mandolini E, Peintner U (2022). Mortierellaceae from subalpine and alpine habitats: new species of Entomortierella, Linnemannia, Mortierella, Podila and Tyroliella gen. nov. Studies in Mycology 103: 25-58. doi: 10.3114/sim.2022.103.02.
Keywords: Mucoromycota systematics and taxonomy; multi-gene phylogeny; new taxa; systematics.
© 2022 Westerdijk Fungal Biodiversity Institute.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
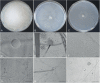



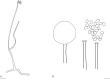
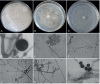
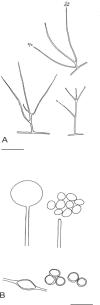

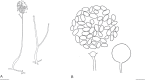















References
-
- Coemans E. (1863). Quelques Hyphomycètes nouveaux. 1. notice: Mortierella polycephala et Martinella pectinata. Bulletin de l’Académie Royale des Sciences de Belgique Classe des Sciences 15: 536-540.
-
- Degawa Y, Gams W. (2004). A new species of Mortierella, and an associated sporangiiferous mycoparasite in a new genus, Nothadelphia. Studies in Mycology 50: 567–572.
Grants and funding
LinkOut - more resources
Full Text Sources
