Colorectal polyps increase the glycolytic activity
- PMID: 37342183
- PMCID: PMC10277630
- DOI: 10.3389/fonc.2023.1171887
Colorectal polyps increase the glycolytic activity
Abstract
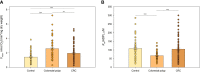
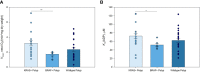
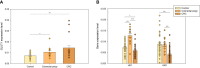
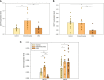
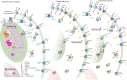
In colorectal cancer (CRC) energy metabolism research, the precancerous stage of polyp has remained rather unexplored. By now, it has been shown that CRC has not fully obtained the glycolytic phenotype proposed by O. Warburg and rather depends on mitochondrial respiration. However, the pattern of metabolic adaptations during tumorigenesis is still unknown. Understanding the interplay between genetic and metabolic changes that initiate tumor development could provide biomarkers for diagnosing cancer early and targets for new cancer therapeutics. We used human CRC and polyp tissue material and performed high-resolution respirometry and qRT-PCR to detect changes on molecular and functional level with the goal of generally describing metabolic reprogramming during CRC development. Colon polyps were found to have a more glycolytic bioenergetic phenotype than tumors and normal tissues. This was supported by a greater GLUT1, HK, LDHA, and MCT expression. Despite the increased glycolytic activity, cells in polyps were still able to maintain a highly functional OXPHOS system. The mechanisms of OXPHOS regulation and the preferred substrates are currently unclear and would require further investigation. During polyp formation, intracellular energy transfer pathways become rearranged mainly by increasing the expression of mitochondrial adenylate kinase (AK) and creatine kinase (CK) isoforms. Decreased glycolysis and maintenance of OXPHOS activity, together with the downregulation of the CK system and the most common AK isoforms (AK1 and AK2), seem to play a relevant role in CRC development.
Keywords: OXPHOS; Warburg effect; colonic adenoma; colorectal cancer; energy metabolism; metabolic phenotype.
Copyright © 2023 Rebane-Klemm, Reinsalu, Puurand, Shevchuk, Bogovskaja, Suurmaa, Valvere, Moreno-Sanchez and Kaambre.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

