Different priming strategies improve distinct therapeutic capabilities of mesenchymal stromal/stem cells: Potential implications for their clinical use
- PMID: 37342218
- PMCID: PMC10277962
- DOI: 10.4252/wjsc.v15.i5.400
Different priming strategies improve distinct therapeutic capabilities of mesenchymal stromal/stem cells: Potential implications for their clinical use
Abstract
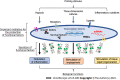
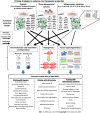
Mesenchymal stromal/stem cells (MSCs) have shown significant therapeutic potential, and have therefore been extensively investigated in preclinical studies of regenerative medicine. However, while MSCs have been shown to be safe as a cellular treatment, they have usually been therapeutically ineffective in human diseases. In fact, in many clinical trials it has been shown that MSCs have moderate or poor efficacy. This inefficacy appears to be ascribable primarily to the heterogeneity of MSCs. Recently, specific priming strategies have been used to improve the therapeutic properties of MSCs. In this review, we explore the literature on the principal priming approaches used to enhance the preclinical inefficacy of MSCs. We found that different priming strategies have been used to direct the therapeutic effects of MSCs toward specific pathological processes. Particularly, while hypoxic priming can be used primarily for the treatment of acute diseases, inflammatory cytokines can be used mainly to prime MSCs in order to treat chronic immune-related disorders. The shift in approach from regeneration to inflammation implies, in MSCs, a shift in the production of functional factors that stimulate regenerative or anti-inflammatory pathways. The opportunity to fine-tune the therapeutic properties of MSCs through different priming strategies could conceivably pave the way for optimizing their therapeutic potential.
Keywords: Hypoxic priming, 3D culture priming; Mesenchymal stromal/stem cell paracrine effects; Mesenchymal stromal/stem cell priming; Mesenchymal stromal/stem cell therapeutic properties; Mesenchymal stromal/stem cells; Pro-inflammatory priming.
©The Author(s) 2023. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures

References
-
- Cittadini E, Brucculeri AM, Quartararo F, Vaglica R, Miceli V, Conaldi PG. Stem cell therapy in the treatment of organic and dysfunctional endometrial pathology. Minerva Obstet Gynecol. 2022;74:504–515. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

