Whole-Genome Doubling as a source of cancer: how, when, where, and why?
- PMID: 37342233
- PMCID: PMC10277508
- DOI: 10.3389/fcell.2023.1209136
Whole-Genome Doubling as a source of cancer: how, when, where, and why?
Abstract
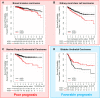
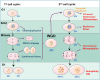
Chromosome instability is a well-known hallmark of cancer, leading to increased genetic plasticity of tumoral cells, which favors cancer aggressiveness, and poor prognosis. One of the main sources of chromosomal instability are events that lead to a Whole-Genome Duplication (WGD) and the subsequently generated cell polyploidy. In recent years, several studies showed that WGD occurs at the early stages of cell transformation, which allows cells to later become aneuploid, thus leading to cancer progression. On the other hand, other studies convey that polyploidy plays a tumor suppressor role, by inducing cell cycle arrest, cell senescence, apoptosis, and even prompting cell differentiation, depending on the tissue cell type. There is still a gap in understanding how cells that underwent WGD can overcome the deleterious effect on cell fitness and evolve to become tumoral. Some laboratories in the chromosomal instability field recently explored this paradox, finding biomarkers that modulate polyploid cells to become oncogenic. This review brings a historical view of how WGD and polyploidy impact cell fitness and cancer progression, and bring together the last studies that describe the genes helping cells to adapt to polyploidy.
Keywords: aneuploidy; chromosomal instability; endoreduplication; mitotic slippage; oncogene; polyploidy; whole-genome doubling.
Copyright © 2023 Sanz-Gómez, González-Álvarez, De Las Rivas and de Cárcer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

