Chitosan and Its Structural Modifications for siRNA Delivery
- PMID: 37342385
- PMCID: PMC10278227
- DOI: 10.34172/apb.2023.030
Chitosan and Its Structural Modifications for siRNA Delivery
Abstract
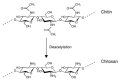
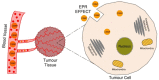
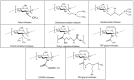
The use of RNA interference mechanism and small interfering RNA (siRNA) in cancer gene therapy is a very promising approach. However, the success of gene silencing is underpinned by the efficient delivery of intact siRNA into the targeted cell. Nowadays, chitosan is one of the most widely studied non-viral vectors for siRNA delivery, since it is a biodegradable, biocompatible and positively charged polymer able to bind to the negatively charged siRNA forming nanoparticles (NPs) that will act as siRNA delivery system. However, chitosan shows several limitations such as low transfection efficiency and low solubility at physiological pH. Therefore, a variety of chemical and non-chemical structural modifications of chitosan were investigated in the attempt to develop a chitosan derivative showing the features of an ideal siRNA carrier. In this review, the most recently proposed chemical modifications of chitosan are outlined. The type of modification, chemical structure, physicochemical properties, siRNA binding affinity and complexation efficiency of the modified chitosan are discussed. Moreover, the resulting NPs characteristics, cellular uptake, serum stability, cytotoxicity and gene transfection efficiency in vitro and/or in vivo are described and compared to the unmodified chitosan. Finally, a critical analysis of a selection of modifications is included, highlighting the most promising ones for this purpose in the future.
Keywords: Chitosan; Chitosan derivatives; Gene therapy; Nanoparticles; Tumour; siRNA.
©2023 The Authors.
Conflict of interest statement
The authors declare no conflict of interest.
References
-
- Mahmoudpour M, Ding S, Lyu Z, Ebrahimi G, Du D, Ezzati Nazhad Dolatabadi J, et al. Aptamer functionalized nanomaterials for biomedical applications: recent advances and new horizons. Nano Today. 2021;39:101177. doi: 10.1016/j.nantod.2021.101177. - DOI
-
- Schiffelers RM, Mastrobattista E. Oligonucleotides. In: Crommelin DJ, Sindelar RD, Meibohm B, eds. Pharmaceutical Biotechnology: Fundamentals and Applications. 3rd ed. New York: Informa Healthcare USA, Inc; 2008. p. 211-24. 10.3109/9781420044386. - DOI
Publication types
LinkOut - more resources
Full Text Sources