Severe Hepatopathy in National Wilms Tumor Studies 3-5: Prevalence, Clinical Features, and Outcomes After Reintroduction of Chemotherapy
- PMID: 37343199
- PMCID: PMC10852371
- DOI: 10.1200/JCO.22.02555
Severe Hepatopathy in National Wilms Tumor Studies 3-5: Prevalence, Clinical Features, and Outcomes After Reintroduction of Chemotherapy
Abstract
Purpose: The safety of reintroducing chemotherapy in the pediatric renal tumor setting after severe hepatopathy (SH), including sinusoidal obstruction syndrome (SOS), is uncertain. We describe the incidence, severity, outcomes, and impact on subsequent treatment for patients with SH from National Wilms Tumor Study (NWTS) protocols 3-5.
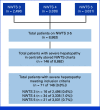
Patients and methods: Archived charts for patients enrolled on NWTS 3-5 who met study inclusion criteria for SH by using established hepatopathy grading scales and clinical criteria were reviewed for demographics, tumor characteristics, radio- and chemotherapy details, SH-related dose modifications, and oncologic outcomes. Genomic analysis for candidate polymorphisms associated with SH was performed in 14 patients.
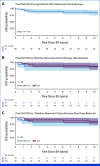
Results: Seventy-one of 8,862 patients (0.8%) met study inclusion criteria. The median time from therapy initiation to SH was 51 days (range, 2-293 days). Sixty percent received radiotherapy, and 56% had right-sided tumors. Grade 1-4 thrombocytopenia was noted in 70% at initial occurrence of SH (median 22,000/microliter). Among 69 of 71 children with SH occurring before the end of therapy (EOT) and post-SH treatment information available, chemotherapy was delayed posthepatopathy for 65% (69% of these at a reduced dose), continued without delay for 20% (57% of these at reduced dose), and stopped completely for 15% (4 of 10 of whom died of SH). Overall, 42% of patients with dose reductions achieved full dose by EOT. The five-year post-SH event-free survival for patients who continued therapy was 89% (95% CI, 81 to 98), with no significant differences by whether delay or dose reduction occurred. We identified no SH-associated pharmacogenomic polymorphism.
Conclusion: The incidence of SH on NWTS 3-5 was low; many had associated severe thrombocytopenia. Careful reintroduction of chemotherapy appeared to be feasible for the majority of patients who developed severe chemotherapy- and/or radiotherapy-induced liver toxicity.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures

References
-
- DeLeve LD, Shulman HM, McDonald GB. Toxic injury to hepatic sinusoids: Sinusoidal obstruction syndrome (veno-occlusive disease) Semin Liver Dis. 2002;22:027–042. - PubMed
-
- Cesaro S, Spiller M, Sartori MT, et al. Veno-occlusive disease in pediatric patients affected by Wilms tumor. Pediatr Blood Cancer. 2011;57:258–261. - PubMed
-
- D'Antiga L, Baker A, Pritchard J, et al. Veno-occlusive disease with multi-organ involvement following actinomycin-D. Eur J Cancer. 2001;37:1141–1148. - PubMed
-
- Jagt CT, Zuckermann M, Ten Kate F, et al. Veno-occlusive disease as a complication of preoperative chemotherapy for Wilms tumor: A clinico-pathological analysis. Pediatr Blood Cancer. 2009;53:1211–1215. - PubMed
-
- Tornesello A, Piciacchia D, Mastrangelo S, et al. Veno-occlusive disease of the liver in right-sided Wilms' tumours. Eur J Cancer. 1998;34:1220–1223. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

