How aberrant N-glycosylation can alter protein functionality and ligand binding: An atomistic view
- PMID: 37343552
- PMCID: PMC10526633
- DOI: 10.1016/j.str.2023.05.017
How aberrant N-glycosylation can alter protein functionality and ligand binding: An atomistic view
Abstract
Protein-assembly defects due to an enrichment of aberrant conformational protein variants are emerging as a new frontier in therapeutics design. Understanding the structural elements that rewire the conformational dynamics of proteins and pathologically perturb functionally oriented ensembles is important for inhibitor development. Chaperones are hub proteins for the assembly of multiprotein complexes and an enrichment of aberrant conformers can affect the cellular proteome, and in turn, phenotypes. Here, we integrate computational and experimental tools to investigte how N-glycosylation of specific residues in glucose-regulated protein 94 (GRP94) modulates internal dynamics and alters the conformational fitness of regions fundamental for the interaction with ATP and synthetic ligands and impacts substructures important for the recognition of interacting proteins. N-glycosylation plays an active role in modulating the energy landscape of GRP94, and we provide support for leveraging the knowledge on distinct glycosylation variants to design molecules targeting GRP94 disease-associated conformational states and assemblies.
Keywords: GRP94; aberrant protein conformations; allostery; disease; drug design; drug selectivity; epichaperomes; functional dynamics; glycosylation; post-translational modifications; protein assembly mutations.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests G. Chiosis, A.R., C.S.D., and P.Y. are inventors on patents covering PU-WS13 and associated composition of matter. G. Chiosis is a founder of Samus Therapeutics.
Figures
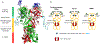




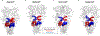



References
-
- Gfeller D, Michielin O, and Zoete V (2013). Shaping the interaction landscape of bioactive molecules. Bioinformatics 29, 3073–3079. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

