G-CSF rescue of FOLFIRINOX-induced neutropenia leads to systemic immune suppression in mice and humans
- PMID: 37344102
- PMCID: PMC10314699
- DOI: 10.1136/jitc-2022-006589
G-CSF rescue of FOLFIRINOX-induced neutropenia leads to systemic immune suppression in mice and humans
Abstract
Background: Recombinant granulocyte colony-stimulating factor (G-CSF) is routinely administered for prophylaxis or treatment of chemotherapy-induced neutropenia. Chronic myelopoiesis and granulopoiesis in patients with cancer has been shown to induce immature monocytes and neutrophils that contribute to both systemic and local immunosuppression in the tumor microenvironment. The effect of recombinant G-CSF (pegfilgrastim or filgrastim) on the production of myeloid-derived suppressive cells is unknown. Here we examined patients with pancreatic cancer, a disease known to induce myeloid-derived suppressor cells (MDSCs), and for which pegfilgrastim is routinely administered concurrently with FOLFIRINOX but not with gemcitabine-based chemotherapy regimens.
Methods: Serial blood was collected from patients with pancreatic ductal adenocarcinoma newly starting on FOLFIRINOX or gemcitabine/n(ab)paclitaxel combination chemotherapy regimens. Neutrophil and monocyte frequencies were determined by flow cytometry from whole blood and peripheral blood mononuclear cell fractions. Serum cytokines were evaluated pretreatment and on-treatment. Patient serum was used in vitro to differentiate healthy donor monocytes to MDSCs as measured by downregulation of major histocompatibility complex II (HLA-DR) and the ability to suppress T-cell proliferation in vitro. C57BL/6 female mice with pancreatic tumors were treated with FOLFIRINOX with or without recombinant G-CSF to directly assess the role of G-CSF on induction of immunosuppressive neutrophils.
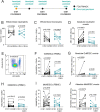
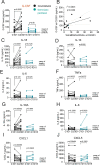
Results: Patients receiving FOLFIRINOX with pegfilgrastim had increased serum G-CSF that correlated with an induction of granulocytic MDSCs. This increase was not observed in patients receiving gemcitabine/n(ab)paclitaxel without pegfilgrastim. Interleukin-18 also significantly increased in serum on FOLFIRINOX treatment. Patient serum could induce MDSCs as determined by in vitro functional assays, and this suppressive effect increased with on-treatment serum. Induction of MDSCs in vitro could be recapitulated by addition of recombinant G-CSF to healthy serum, indicating that G-CSF is sufficient for MDSC differentiation. In mice, neutrophils isolated from spleen of G-CSF-treated mice were significantly more capable of suppressing T-cell proliferation.
Conclusions: Pegfilgrastim use contributes to immune suppression in both humans and mice with pancreatic cancer. These results suggest that use of recombinant G-CSF as supportive care, while critically important for mitigating neutropenia, may complicate efforts to induce antitumor immunity.
Keywords: cytokines; gastrointestinal neoplasms; immunotherapy; myeloid-derived suppressor cells; neutrophil infiltration.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SKD received research funding unrelated to this project from Eli Lilly and Company, Novartis Pharmaceuticals, Genocea, and Bristol-Myers Squibb and is a founder, science advisory board member and equity holder in Kojin. MD has research funding from Eli Lilly; he has received consulting fees from Genentech, ORIC Pharmaceuticals, Partner Therapeutics, SQZ Biotech, AzurRx, Eli Lilly, Mallinckrodt Pharmaceuticals, Aditum, Foghorn Therapeutics, Palleon, and Moderna; and he is a member of the Scientific Advisory Board for Neoleukin Therapeutics, Veravas and Cerberus Therapeutics. HS receives research funding from AstraZeneca and consulting fees from Merck and Dewpoint Therapeutics. DAR is on the scientific advisory board of AxialTx and a consultant for Boston Scientific and Instylla. MBY receives research funding from Janssen Oncology and fees for peer review services from UpToDate. NJM receives research funding from Bristol-Myers Squibb. PCE has received consulting fees from ALX Oncology, Arcus Bioscience, Astellas, AstraZeneca, Blueprint Medicines, Celgene, Coherus, Daiichi-Sankyo, Five Prime, Ideaya, Istari, Legend, Lilly, Loxo, Merck, Novartis, Ono, Servier, Taiho, Takeda, Turning Point, Therapeutics, Xencor, and Zymeworks. KN received research funding from Pharmavite, Evergrande Group, Janssen, Revolution Medicines and is on the SAB or received consulting fees from Bayer, GlaxoSmithKline, and Pfizer. BMW receives consulting fees from Celgene, GRAIL, and Mirati and research support from Celgene, Eli Lilly, Novartis, and Revolution Medicines.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
