Single Ad26.COV2.S booster dose following two doses of BBIBP-CorV vaccine against SARS-CoV-2 infection in adults: Day 28 results of a phase 1/2 open-label trial
- PMID: 37344265
- PMCID: PMC10267503
- DOI: 10.1016/j.vaccine.2023.06.043
Single Ad26.COV2.S booster dose following two doses of BBIBP-CorV vaccine against SARS-CoV-2 infection in adults: Day 28 results of a phase 1/2 open-label trial
Abstract
Background: The inactivated COVID-19 whole-virus vaccine BBIBP-CorV has been extensively used worldwide. Heterologous boosting after primary vaccination can induce higher immune responses against SARS-CoV-2 than homologous boosting. The safety and immunogenicity after 28 days of a single Ad26.COV2.S booster dose given at different intervals after 2 doses of BBIBP-CorV are presented.
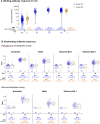
Methods: This open-label phase 1/2 trial was conducted in healthy adults in Thailand who had completed 2-dose primary vaccination with BBIBP-CorV. Participants received a single booster dose of Ad26.COV2.S (5 × 1010 virus particles) 90-240 days (Group A1; n = 360) or 45-75 days (Group A2; n = 66) after the second BBIBP-CorV dose. Safety and immunogenicity were assessed over 28 days. Binding IgG antibodies to the full-length pre-fusion Spike and anti-nucleocapsid proteins of SARS-CoV-2 were measured by enzyme-linked immunosorbent assay. The SARS-CoV-2 pseudovirus neutralization assay and live virus microneutralization assay were used to quantify the neutralizing activity of antibodies against ancestral SARS-CoV-2 (Wuhan-Hu-1) and the delta (B.1.617.2) and omicron (B.1.1.529/BA.1 and BA.2) variants. The cell-mediated immune response was measured using a quantitative interferon (IFN)-γ release assay in whole blood.
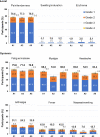
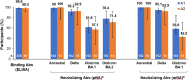
Results: Solicited local and systemic adverse events (AEs) on days 0-7 were mostly mild, as were unsolicited vaccine-related AEs during days 0-28, with no serious AEs. On day 28, anti-Spike binding antibodies increased from baseline by 487- and 146-fold in Groups A1 and A2, and neutralizing antibodies against ancestral SARS-CoV-2 by 55- and 37-fold, respectively. Humoral responses were strongest against ancestral SARS-CoV-2, followed by the delta, then the omicron BA.2 and BA.1 variants. T-cell-produced interferon-γ increased approximately 10-fold in both groups.
Conclusions: A single heterologous Ad26.COV2.S booster dose after two BBIBP-CorV doses was well tolerated and induced robust humoral and cell-mediated immune responses measured at day 28 in both interval groups.
Clinical trials registration: NCT05109559.
Keywords: Ad26.COV2.S; COVID-19; Delta; Heterologous booster; Neutralizing antibodies; Omicron; SARS-CoV-2; Thailand; Variants of concern; Whole inactivated virus vaccine.
Copyright © 2023 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: S.M., R.W., S.K., S.L., A.J., A.T., V.L., P.C., N.T., N.C. report no potential conflicts of interest. S.N. has received honoraria for lectures from GSK, Zuellig Pharma, Astra Zeneca, Novartis, Abbot, Sanofi, Organon and Takeda and has participated in advisory boards for GlaxoSmithKline and Takeda. J.K.L. and T.A.W. and received institutional funding from Mahidol University to support study conduct, and T.A.W. supported management of Data Safety Monitoring Board activities and meetings. J-L.E. has received funding from Mahidol University and Johnson & Johnson for study support, and is the coordinator of the study Data Safety Monitoring Board. M.F.R. is an employee of Janssen Pharmaceuticals and holds restricted stock units in the company. C.L. is an employee of Janssen Pharmaceuticals. T.K.M. has received consulting fees from Janssen Pharmaceuticals. P.P. has received funding from Mahidol University and Johnson & Johnson for study support.
Figures
Similar articles
-
Heterologous Ad26.COV2.S booster after primary BBIBP-CorV vaccination against SARS-CoV-2 infection: 1-year follow-up of a phase 1/2 open-label trial.Vaccine. 2024 Jul 25;42(19):3999-4010. doi: 10.1016/j.vaccine.2024.05.010. Epub 2024 May 13. Vaccine. 2024. PMID: 38744598 Clinical Trial.
-
Immunogenicity and Safety of Heterologous Versus Homologous Prime-Boost Regimens With BBIBP-CorV and Ad26.COV2.S COVID-19 Vaccines: A Multicentric, Randomized, Observer-Blinded Non-inferiority Trial in Madagascar and Mozambique.Clin Infect Dis. 2025 Jul 22;80(Supplement_1):S37-S46. doi: 10.1093/cid/ciaf130. Clin Infect Dis. 2025. PMID: 40694514 Free PMC article. Clinical Trial.
-
Safety, immunogenicity, and efficacy of the mRNA vaccine CS-2034 as a heterologous booster versus homologous booster with BBIBP-CorV in adults aged ≥18 years: a randomised, double-blind, phase 2b trial.Lancet Infect Dis. 2023 Sep;23(9):1020-1030. doi: 10.1016/S1473-3099(23)00199-8. Epub 2023 May 19. Lancet Infect Dis. 2023. PMID: 37216958 Clinical Trial.
-
Immunogenicity and efficacy of Ad26.COV2.S: An adenoviral vector-based COVID-19 vaccine.Immunol Rev. 2022 Sep;310(1):47-60. doi: 10.1111/imr.13088. Epub 2022 Jun 11. Immunol Rev. 2022. PMID: 35689434 Free PMC article. Review.
-
Immunity after COVID-19 vaccination in people with higher risk of compromised immune status: a scoping review.Cochrane Database Syst Rev. 2022 Aug 9;8(8):CD015021. doi: 10.1002/14651858.CD015021. Cochrane Database Syst Rev. 2022. PMID: 35943061 Free PMC article.
Cited by
-
Immunogenicity of BNT162b2 in children 6 months to under 5 years of age with previous SARS-CoV-2 infection, in the era of Omicron predominance.Vaccine X. 2023 Aug 5;15:100367. doi: 10.1016/j.jvacx.2023.100367. eCollection 2023 Dec. Vaccine X. 2023. PMID: 37601322 Free PMC article.
-
Effect of wild-type vaccine doses on BA.5 hybrid immunity, disease severity, and XBB reinfection risk.J Virol. 2024 Dec 17;98(12):e0128524. doi: 10.1128/jvi.01285-24. Epub 2024 Nov 5. J Virol. 2024. PMID: 39499071 Free PMC article.
-
A Comparative Study of Immunogenicity, Antibody Persistence, and Safety of Three Different COVID-19 Boosters between Individuals with Comorbidities and the Normal Population.Vaccines (Basel). 2023 Aug 17;11(8):1376. doi: 10.3390/vaccines11081376. Vaccines (Basel). 2023. PMID: 37631944 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

