Acentrosomal spindles assemble from branching microtubule nucleation near chromosomes in Xenopus laevis egg extract
- PMID: 37344488
- PMCID: PMC10284841
- DOI: 10.1038/s41467-023-39041-z
Acentrosomal spindles assemble from branching microtubule nucleation near chromosomes in Xenopus laevis egg extract
Abstract
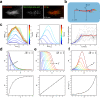
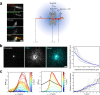
Microtubules are generated at centrosomes, chromosomes, and within spindles during cell division. Whereas microtubule nucleation at the centrosome is well characterized, much remains unknown about where, when, and how microtubules are nucleated at chromosomes. To address these questions, we reconstitute microtubule nucleation from purified chromosomes in meiotic Xenopus egg extract and find that chromosomes alone can form spindles. We visualize microtubule nucleation near chromosomes using total internal reflection fluorescence microscopy to find that this occurs through branching microtubule nucleation. By inhibiting molecular motors, we find that the organization of the resultant polar branched networks is consistent with a theoretical model where the effectors for branching nucleation are released by chromosomes, forming a concentration gradient that spatially biases branching microtbule nucleation. In the presence of motors, these branched networks are ultimately organized into functional spindles, where the number of emergent spindle poles scales with the number of chromosomes and total chromatin area.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Visualizing and Analyzing Branching Microtubule Nucleation Using Meiotic Xenopus Egg Extracts and TIRF Microscopy.Methods Mol Biol. 2016;1413:77-85. doi: 10.1007/978-1-4939-3542-0_6. Methods Mol Biol. 2016. PMID: 27193844 Free PMC article.
-
Multiple motors cooperate to establish and maintain acentrosomal spindle bipolarity in C. elegans oocyte meiosis.Elife. 2022 Feb 11;11:e72872. doi: 10.7554/eLife.72872. Elife. 2022. PMID: 35147496 Free PMC article.
-
Augmin promotes meiotic spindle formation and bipolarity in Xenopus egg extracts.Proc Natl Acad Sci U S A. 2011 Aug 30;108(35):14473-8. doi: 10.1073/pnas.1110412108. Epub 2011 Aug 15. Proc Natl Acad Sci U S A. 2011. PMID: 21844347 Free PMC article.
-
Focusing on spindle poles.J Cell Sci. 1998 Jun;111 ( Pt 11):1477-81. doi: 10.1242/jcs.111.11.1477. J Cell Sci. 1998. PMID: 9580556 Review.
-
Role of chromosomes in assembly of meiotic and mitotic spindles.Prog Cell Cycle Res. 1997;3:271-84. doi: 10.1007/978-1-4615-5371-7_22. Prog Cell Cycle Res. 1997. PMID: 9552422 Review.
Cited by
-
HURP facilitates spindle assembly by stabilizing microtubules and working synergistically with TPX2.Nat Commun. 2024 Nov 8;15(1):9689. doi: 10.1038/s41467-024-53630-6. Nat Commun. 2024. PMID: 39516491 Free PMC article.
-
Microtubule nucleation for spindle assembly: one molecule at a time.Trends Biochem Sci. 2023 Sep;48(9):761-775. doi: 10.1016/j.tibs.2023.06.004. Epub 2023 Jul 21. Trends Biochem Sci. 2023. PMID: 37482516 Free PMC article. Review.
-
Augmin is a Ran-regulated spindle assembly factor.J Biol Chem. 2023 Jun;299(6):104736. doi: 10.1016/j.jbc.2023.104736. Epub 2023 Apr 21. J Biol Chem. 2023. PMID: 37086784 Free PMC article.
-
Building on-chip cytoskeletal circuits via branched microtubule networks.Proc Natl Acad Sci U S A. 2024 Jan 23;121(4):e2315992121. doi: 10.1073/pnas.2315992121. Epub 2024 Jan 17. Proc Natl Acad Sci U S A. 2024. PMID: 38232292 Free PMC article.
-
HURP facilitates spindle assembly by stabilizing microtubules and working synergistically with TPX2.bioRxiv [Preprint]. 2023 Dec 18:2023.12.18.571906. doi: 10.1101/2023.12.18.571906. bioRxiv. 2023. Update in: Nat Commun. 2024 Nov 8;15(1):9689. doi: 10.1038/s41467-024-53630-6. PMID: 38187686 Free PMC article. Updated. Preprint.
References
-
- McKim, K. S. & Hawley, R. S. Chromosomal control of meiotic cell division. Science10.1126/science.270.5242.1595 (1995). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

