Structure and function of the RAD51B-RAD51C-RAD51D-XRCC2 tumour suppressor
- PMID: 37344587
- PMCID: PMC7614784
- DOI: 10.1038/s41586-023-06179-1
Structure and function of the RAD51B-RAD51C-RAD51D-XRCC2 tumour suppressor
Abstract
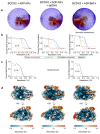
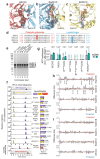
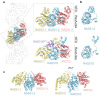
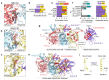
Homologous recombination is a fundamental process of life. It is required for the protection and restart of broken replication forks, the repair of chromosome breaks and the exchange of genetic material during meiosis. Individuals with mutations in key recombination genes, such as BRCA2 (also known as FANCD1), or the RAD51 paralogues RAD51B, RAD51C (also known as FANCO), RAD51D, XRCC2 (also known as FANCU) and XRCC3, are predisposed to breast, ovarian and prostate cancers1-10 and the cancer-prone syndrome Fanconi anaemia11-13. The BRCA2 tumour suppressor protein-the product of BRCA2-is well characterized, but the cellular functions of the RAD51 paralogues remain unclear. Genetic knockouts display growth defects, reduced RAD51 focus formation, spontaneous chromosome abnormalities, sensitivity to PARP inhibitors and replication fork defects14,15, but the precise molecular roles of RAD51 paralogues in fork stability, DNA repair and cancer avoidance remain unknown. Here we used cryo-electron microscopy, AlphaFold2 modelling and structural proteomics to determine the structure of the RAD51B-RAD51C-RAD51D-XRCC2 complex (BCDX2), revealing that RAD51C-RAD51D-XRCC2 mimics three RAD51 protomers aligned within a nucleoprotein filament, whereas RAD51B is highly dynamic. Biochemical and single-molecule analyses showed that BCDX2 stimulates the nucleation and extension of RAD51 filaments-which are essential for recombinational DNA repair-in reactions that depend on the coupled ATPase activities of RAD51B and RAD51C. Our studies demonstrate that BCDX2 orchestrates RAD51 assembly on single stranded DNA for replication fork protection and double strand break repair, in reactions that are critical for tumour avoidance.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
S.J.B. is a co-founder and VP Science Strategy at Artios Pharma Ltd., Babraham Research Campus, U.K. Other authors declare no conflict of interest.
Figures











References
-
- Venkitaraman AR. Cancer suppression by the chromosome custodians, BRCA1 and BRCA2. Science. 2014;343:1470–1475. - PubMed
-
- Meindl A, et al. Germline mutations in breast and ovarian cancer pedigrees establish RAD51C as a human cancer susceptibility gene. Nat Genet. 2010;42:410–414. - PubMed
-
- Pelttari LM, et al. RAD51C is a susceptibility gene for ovarian cancer. Hum Mol Genet. 2011;20:3278–3288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

