Signalling by senescent melanocytes hyperactivates hair growth
- PMID: 37344645
- PMCID: PMC10284692
- DOI: 10.1038/s41586-023-06172-8
Signalling by senescent melanocytes hyperactivates hair growth
Abstract
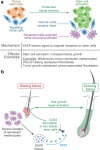
Niche signals maintain stem cells in a prolonged quiescence or transiently activate them for proper regeneration1. Altering balanced niche signalling can lead to regenerative disorders. Melanocytic skin nevi in human often display excessive hair growth, suggesting hair stem cell hyperactivity. Here, using genetic mouse models of nevi2,3, we show that dermal clusters of senescent melanocytes drive epithelial hair stem cells to exit quiescence and change their transcriptome and composition, potently enhancing hair renewal. Nevus melanocytes activate a distinct secretome, enriched for signalling factors. Osteopontin, the leading nevus signalling factor, is both necessary and sufficient to induce hair growth. Injection of osteopontin or its genetic overexpression is sufficient to induce robust hair growth in mice, whereas germline and conditional deletions of either osteopontin or CD44, its cognate receptor on epithelial hair cells, rescue enhanced hair growth induced by dermal nevus melanocytes. Osteopontin is overexpressed in human hairy nevi, and it stimulates new growth of human hair follicles. Although broad accumulation of senescent cells, such as upon ageing or genotoxic stress, is detrimental for the regenerative capacity of tissue4, we show that signalling by senescent cell clusters can potently enhance the activity of adjacent intact stem cells and stimulate tissue renewal. This finding identifies senescent cells and their secretome as an attractive therapeutic target in regenerative disorders.
© 2023. The Author(s).
Conflict of interest statement
M.V.P. is an inventor on a patent application filed by the University of California, Irvine describing the use of senescent secretome factors for promoting hair growth, among other claims. M.V.P. is also a co-founder and Chief Scientific Officer at the Amplifica Holdings Group, Inc. N.U.S. is employed by the Amplifica Holdings Group, Inc. All other authors declare no competing interests.
Figures













References
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR079150/AR/NIAMS NIH HHS/United States
- R01 AR044882/AR/NIAMS NIH HHS/United States
- T32 CA009054/CA/NCI NIH HHS/United States
- R01 CA244571/CA/NCI NIH HHS/United States
- R33 AR073031/AR/NIAMS NIH HHS/United States
- T32 AR080622/AR/NIAMS NIH HHS/United States
- U54 CA217378/CA/NCI NIH HHS/United States
- R01 AR079470/AR/NIAMS NIH HHS/United States
- R61 AR073031/AR/NIAMS NIH HHS/United States
- R21 AR078939/AR/NIAMS NIH HHS/United States
- U01 AR073159/AR/NIAMS NIH HHS/United States
- P30 AR075047/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

