Integrative multi-omics analysis reveals novel idiopathic pulmonary fibrosis endotypes associated with disease progression
- PMID: 37344825
- PMCID: PMC10283254
- DOI: 10.1186/s12931-023-02435-0
Integrative multi-omics analysis reveals novel idiopathic pulmonary fibrosis endotypes associated with disease progression
Abstract
Background: Idiopathic pulmonary fibrosis (IPF) is characterized by the accumulation of extracellular matrix in the pulmonary interstitium and progressive functional decline. We hypothesized that integration of multi-omics data would identify clinically meaningful molecular endotypes of IPF.
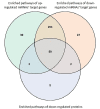
Methods: The IPF-PRO Registry is a prospective registry of patients with IPF. Proteomic and transcriptomic (including total RNA [toRNA] and microRNA [miRNA]) analyses were performed using blood collected at enrollment. Molecular data were integrated using Similarity Network Fusion, followed by unsupervised spectral clustering to identify molecular subtypes. Cox proportional hazards models tested the relationship between these subtypes and progression-free and transplant-free survival. The molecular subtypes were compared to risk groups based on a previously described 52-gene (toRNA expression) signature. Biological characteristics of the molecular subtypes were evaluated via linear regression differential expression and canonical pathways (Ingenuity Pathway Analysis [IPA]) over-representation analyses.
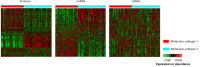
Results: Among 232 subjects, two molecular subtypes were identified. Subtype 1 (n = 105, 45.3%) and Subtype 2 (n = 127, 54.7%) had similar distributions of age (70.1 +/- 8.1 vs. 69.3 +/- 7.6 years; p = 0.31) and sex (79.1% vs. 70.1% males, p = 0.16). Subtype 1 had more severe disease based on composite physiologic index (CPI) (55.8 vs. 51.2; p = 0.002). After adjusting for CPI and antifibrotic treatment at enrollment, subtype 1 experienced shorter progression-free survival (HR 1.79, 95% CI 1.28,2.56; p = 0.0008) and similar transplant-free survival (HR 1.30, 95% CI 0.87,1.96; p = 0.20) as subtype 2. There was little agreement in the distribution of subjects to the molecular subtypes and the risk groups based on 52-gene signature (kappa = 0.04, 95% CI= -0.08, 0.17), and the 52-gene signature risk groups were associated with differences in transplant-free but not progression-free survival. Based on heatmaps and differential expression analyses, proteins and miRNAs (but not toRNA) contributed to classification of subjects to the molecular subtypes. The IPA showed enrichment in pulmonary fibrosis-relevant pathways, including mTOR, VEGF, PDGF, and B-cell receptor signaling.
Conclusions: Integration of transcriptomic and proteomic data from blood enabled identification of clinically meaningful molecular endotypes of IPF. If validated, these endotypes could facilitate identification of individuals likely to experience disease progression and enrichment of clinical trials.
Trial registration: NCT01915511.
Keywords: Biomarkers; Cluster analysis; Computational biology; Lung fibrosis; Proteomics; RNA.
© 2023. The Author(s).
Conflict of interest statement
Peifeng Ruan, Hongyu Zhao and Mary Porteous have nothing to report. Jamie L Todd, Megan L Neely and Scott M Palmer are employees of the Duke Clinical Research Institute (DCRI) which receives funding support from Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI) to coordinate the IPF-PRO/ILD-PRO Registry. Jamie L Todd also reports grants from AstraZeneca, BI, CareDx and has participated on Data Safety Monitoring Boards or Advisory Boards for Altavant Sciences, Natera, Theravance. Megan L Neely also reports honoraria for a lecture from North Carolina State University. Yi Liu, Richard Vinisko, Julia F Soellner, Ramona Schmid, Christian Hesslinger and Thomas B Leonard are employees of BI. Robert J Kaner reports grants paid to his institution from Bellerophon, BI, Genentech, the National Institutes of Health, Respivant, Toray, the US Department of Defense; royalties or licenses from UpToDate; consulting fees from AstraZeneca and Galapagos; speaker fees from BI, the France Foundation, Genentech, Vindico; has participated on Data Safety Monitoring Boards or Advisory Boards for BI, Genentech, Pliant, PureTech; holds unpaid leadership or fiduciary roles with the Pulmonary Wellness Foundation and the Stony Wold Foundation; holds stock or stock options with Air Cycle Systems and Doximity; and has received medical writing support from AstraZeneca, BI, Galapagos, Genentech. Tracy R Luckhardt reports grants from Bellerophon and FibroGen for her role as a clinical trial investigator. Imre Noth reports a contract for biospecimens from Veracyte; royalties from UpToDate; consulting fees from BI, Genentech, Sanofi; he holds a patent for a gene signature predictor of FVC and has a PCSK6 patent pending; he holds licenses for protein markers in IPF; he was a member of the Yale COVID Data Safety Monitoring Board. Rishi Raj has received an investigator-initiated research grant from BI and served on an advisory board for BI. Zeenat Safdar reports consulting fees and honoraria for lectures from BI and Genentech. Mary E Strek has served as Principal Investigator for institutional and investigator-initiated grants from BI, and for institutional grants from Galapagos, Novartis, the Pulmonary Fibrosis Foundation; received honoraria for lectures and a textbook from CHEST; served on an adjudication committee for FibroGen; is on the American Thoracic Society Clinical Problems Committee and Research Innovation Summit Planning Committee; is Chair of the PFF Registry Scientific Review Committee. Scott M Palmer reports research funding to Duke/DCRI from AstraZeneca, Bristol Myers Squibb, CareDx; royalties or licenses from UpToDate; and speaker fees from Altavant Sciences, Bristol Myers Squibb, Mereo Biopharma, Theravance. Margaret L Salisbury reports grants paid to her institution from the National Institutes of Health; consulting fees from BI, Orinove Inc., Roche; payment for lectures from BI; and reimbursement to attend an investigators’ meeting from BI.
Figures



References
-
- Raghu G, Remy-Jardin M, Richeldi L, Thomson CC, Inoue Y, Johkoh T, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2022;205(9):e18–47. doi: 10.1164/rccm.202202-0399ST. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

