DHCR24 reverses Alzheimer's disease-related pathology and cognitive impairment via increasing hippocampal cholesterol levels in 5xFAD mice
- PMID: 37344916
- PMCID: PMC10286507
- DOI: 10.1186/s40478-023-01593-y
DHCR24 reverses Alzheimer's disease-related pathology and cognitive impairment via increasing hippocampal cholesterol levels in 5xFAD mice
Abstract
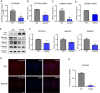
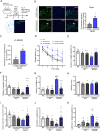
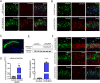
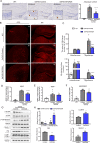
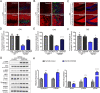
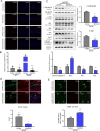
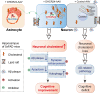
Accumulating evidences reveal that cellular cholesterol deficiency could trigger the onset of Alzheimer's disease (AD). As a key regulator, 24-dehydrocholesterol reductase (DHCR24) controls cellular cholesterol homeostasis, which was found to be downregulated in AD vulnerable regions and involved in AD-related pathological activities. However, DHCR24 as a potential therapeutic target for AD remains to be identified. In present study, we demonstrated the role of DHCR24 in AD by employing delivery of adeno-associated virus carrying DHCR24 gene into the hippocampus of 5xFAD mice. Here, we found that 5xFAD mice had lower levels of cholesterol and DHCR24 expression, and the cholesterol loss was alleviated by DHCR24 overexpression. Surprisingly, the cognitive impairment of 5xFAD mice was significantly reversed after DHCR24-based gene therapy. Moreover, we revealed that DHCR24 knock-in successfully prevented or reversed AD-related pathology in 5xFAD mice, including amyloid-β deposition, synaptic injuries, autophagy, reactive astrocytosis, microglial phagocytosis and apoptosis. In conclusion, our results firstly demonstrated that the potential value of DHCR24-mediated regulation of cellular cholesterol level as a promising treatment for AD.
Keywords: 24-dehydrocholesterol reductase (DHCR24); Alzheimer’s disease; Cholesterol; Gene therapy; Neurodegeneration; Neuroprotection; Pathogenesis.
© 2023. The Author(s).
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures
Similar articles
-
Cellular Cholesterol Loss Impairs Synaptic Vesicle Mobility via the CAMK2/Synapsin-1 Signaling Pathway.Front Biosci (Landmark Ed). 2025 Jan 20;30(1):27111. doi: 10.31083/FBL27111. Front Biosci (Landmark Ed). 2025. PMID: 39862102
-
The role of DHCR24 in the pathogenesis of AD: re-cognition of the relationship between cholesterol and AD pathogenesis.Acta Neuropathol Commun. 2022 Mar 16;10(1):35. doi: 10.1186/s40478-022-01338-3. Acta Neuropathol Commun. 2022. PMID: 35296367 Free PMC article. Review.
-
DHCR24 Knockdown Induces Tau Hyperphosphorylation at Thr181, Ser199, Ser262, and Ser396 Sites via Activation of the Lipid Raft-Dependent Ras/MEK/ERK Signaling Pathway in C8D1A Astrocytes.Mol Neurobiol. 2022 Sep;59(9):5856-5873. doi: 10.1007/s12035-022-02945-w. Epub 2022 Jul 8. Mol Neurobiol. 2022. PMID: 35804281 Free PMC article.
-
Apolipoprotein E4 interferes with lipid metabolism to exacerbate depression-like behaviors in 5xFAD mice.Animal Model Exp Med. 2024 Jun;7(3):347-361. doi: 10.1002/ame2.12446. Epub 2024 Jun 19. Animal Model Exp Med. 2024. PMID: 38895818 Free PMC article.
-
Desmosterol and DHCR24: unexpected new directions for a terminal step in cholesterol synthesis.Prog Lipid Res. 2013 Oct;52(4):666-80. doi: 10.1016/j.plipres.2013.09.002. Epub 2013 Oct 2. Prog Lipid Res. 2013. PMID: 24095826 Review.
Cited by
-
The impact of aging and oxidative stress in metabolic and nervous system disorders: programmed cell death and molecular signal transduction crosstalk.Front Immunol. 2023 Nov 8;14:1273570. doi: 10.3389/fimmu.2023.1273570. eCollection 2023. Front Immunol. 2023. PMID: 38022638 Free PMC article. Review.
-
Cognitive Impairment in Multiple Sclerosis.Bioengineering (Basel). 2023 Jul 23;10(7):871. doi: 10.3390/bioengineering10070871. Bioengineering (Basel). 2023. PMID: 37508898 Free PMC article. Review.
-
High mRNA Expression of 24 Dehydrocholesterol Reductase (DHCR24) in the Treatment of Doxorubicin-Induced Heart Failure in Rats.Int J Mol Sci. 2025 Jan 1;26(1):312. doi: 10.3390/ijms26010312. Int J Mol Sci. 2025. PMID: 39796168 Free PMC article.
-
DHCR24 in Tumor Diagnosis and Treatment: A Comprehensive Review.Technol Cancer Res Treat. 2024 Jan-Dec;23:15330338241259780. doi: 10.1177/15330338241259780. Technol Cancer Res Treat. 2024. PMID: 38847653 Free PMC article. Review.
-
Therapeutic Strategies Aimed at Improving Neuroplasticity in Alzheimer Disease.Pharmaceutics. 2023 Jul 31;15(8):2052. doi: 10.3390/pharmaceutics15082052. Pharmaceutics. 2023. PMID: 37631266 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

