Loss of the Secretin Receptor Impairs Renal Bicarbonate Excretion and Aggravates Metabolic Alkalosis in Mice during Acute Base-Loading
- PMID: 37344929
- PMCID: PMC10400107
- DOI: 10.1681/ASN.0000000000000173
Loss of the Secretin Receptor Impairs Renal Bicarbonate Excretion and Aggravates Metabolic Alkalosis in Mice during Acute Base-Loading
Abstract
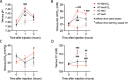
Significance statement: During acute base excess, the renal collecting duct β -intercalated cells ( β -ICs) become activated to increase urine base excretion. This process is dependent on pendrin and cystic fibrosis transmembrane regulator (CFTR) expressed in the apical membrane of β -ICs. The signal that leads to activation of this process was unknown. Plasma secretin levels increase during acute alkalosis, and the secretin receptor (SCTR) is functionally expressed in β -ICs. We find that mice with global knockout for the SCTR lose their ability to acutely increase renal base excretion. This forces the mice to lower their ventilation to cope with this challenge. Our findings suggest that secretin is a systemic bicarbonate-regulating hormone, likely being released from the small intestine during alkalosis.
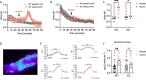
Background: The secretin receptor (SCTR) is functionally expressed in the basolateral membrane of the β -intercalated cells of the kidney cortical collecting duct and stimulates urine alkalization by activating the β -intercalated cells. Interestingly, the plasma secretin level increases during acute metabolic alkalosis, but its role in systemic acid-base homeostasis was unclear. We hypothesized that the SCTR system is essential for renal base excretion during acute metabolic alkalosis.
Methods: We conducted bladder catheterization experiments, metabolic cage studies, blood gas analysis, barometric respirometry, perfusion of isolated cortical collecting ducts, immunoblotting, and immunohistochemistry in SCTR wild-type and knockout (KO) mice. We also perfused isolated rat small intestines to study secretin release.
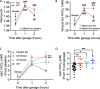
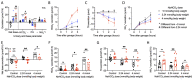
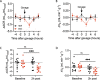
Results: In wild-type mice, secretin acutely increased urine pH and pendrin function in isolated perfused cortical collecting ducts. These effects were absent in KO mice, which also did not sufficiently increase renal base excretion during acute base loading. In line with these findings, KO mice developed prolonged metabolic alkalosis when exposed to acute oral or intraperitoneal base loading. Furthermore, KO mice exhibited transient but marked hypoventilation after acute base loading. In rats, increased blood alkalinity of the perfused upper small intestine increased venous secretin release.
Conclusions: Our results suggest that loss of SCTR impairs the appropriate increase of renal base excretion during acute base loading and that SCTR is necessary for acute correction of metabolic alkalosis. In addition, our findings suggest that blood alkalinity increases secretin release from the small intestine and that secretin action is critical for bicarbonate homeostasis.
Copyright © 2023 by the American Society of Nephrology.
Conflict of interest statement
P Berg reports Patents or Royalties inventor in two patent applications filed by Aarhus University, Denmark One describes the use of urinary bicarbonate excretion as a biomarker in cystic fibrosis, the second describes the use of urinary acid–base markers as a prognostic marker and companion diagnostic in patients with CKD J Leipziger reports the following: Ownership Interest Osram Siemens, and Siemens Energy All remaining authors have nothing to disclose S Svendsen reports Patents or Royalties Patent on employing a urine acid base score as guideline for base supplementation in chronic kidney disease (5% ownership) J Holst reports Consultancy: JJH Novo Nordisk, Merck/MSD Spouse Mette M Rosenkilde (MMR) Alphasights Eli Lilly Shouti/Structure TX Zealand Pharma Alcimed Novo Nordisk Ownership Interest: JJH/MMR Bainan Biotech Antag Therapeutics Research Funding Scohia Honoraria Novo Nordisk Merck/MSD Advisory or Leadership Role JJH Novo Nordisk Eli Lilly Zealand pharma Speakers Bureau Novo Nordisk Novo Nordisk Pharma Novo Nordisk Scandinavia AB and Mayo Clinic and Other Interests or Relationships JJH Member of the Incretin EASD Study Group.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

