The Role of TRAIL in Apoptosis and Immunosurveillance in Cancer
- PMID: 37345089
- PMCID: PMC10216286
- DOI: 10.3390/cancers15102752
The Role of TRAIL in Apoptosis and Immunosurveillance in Cancer
Abstract
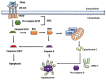
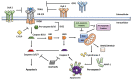
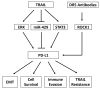
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is a member of the TNF superfamily that selectively induces apoptosis in tumor cells without harming normal cells, making it an attractive agent for cancer therapy. TRAIL induces apoptosis by binding to and activating its death receptors DR4 and DR5. Several TRAIL-based treatments have been developed, including recombinant forms of TRAIL and its death receptor agonist antibodies, but the efficacy of TRAIL-based therapies in clinical trials is modest. In addition to inducing cancer cell apoptosis, TRAIL is expressed in immune cells and plays a critical role in tumor surveillance. Emerging evidence indicates that the TRAIL pathway may interact with immune checkpoint proteins, including programmed death-ligand 1 (PD-L1), to modulate PD-L1-based tumor immunotherapies. Therefore, understanding the interaction between TRAIL and the immune checkpoint PD-L1 will lead to the development of new strategies to improve TRAIL- and PD-L1-based therapies. This review discusses recent findings on TRAIL-based therapy, resistance, and its involvement in tumor immunosurveillance.
Keywords: PD-L1; TRAIL; apoptosis; immunosurveillance; resistance.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Wiley S.R., Schooley K., Smolak P.J., Din W.S., Huang C.P., Nicholl J.K., Sutherland G.R., Smith T.D., Rauch C., Smith C.A., et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

