Boron Neutron Capture Therapy Followed by Image-Guided Intensity-Modulated Radiotherapy for Locally Recurrent Head and Neck Cancer: A Prospective Phase I/II Trial
- PMID: 37345099
- PMCID: PMC10216174
- DOI: 10.3390/cancers15102762
Boron Neutron Capture Therapy Followed by Image-Guided Intensity-Modulated Radiotherapy for Locally Recurrent Head and Neck Cancer: A Prospective Phase I/II Trial
Abstract
Background: This trial investigated the efficacy and safety of salvage boron neutron capture therapy (BNCT) combined with image-guided intensity-modulated radiotherapy (IG-IMRT) for recurrent head and neck cancer after prior radiotherapy (RT).
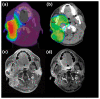
Methods: BNCT was administered using an intravenous boronophenylalanine-fructose complex (500 mg/kg) in a single fraction; multifractionated IG-IMRT was administered 28 days after BNCT. For BNCT, the mucosa served as the dose-limiting organ. For IG-IMRT, the clinical target volume (CTV) and the planning target volume (PTV) were generated according to the post-BNCT gross tumor volume (GTV) with chosen margins.
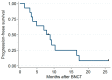
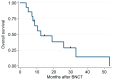
Results: This trial enrolled 14 patients, and 12 patients received combined treatment. The median BNCT average dose for the GTV was 21.6 Gy-Eq, and the median IG-IMRT dose for the PTV was 46.8 Gy/26 fractions. After a median (range) follow-up period of 11.8 (3.6 to 53.2) months, five patients had a complete response and four had a partial response. One patient had grade 4 laryngeal edema; another patient had a grade 4 hemorrhage. Most tumor progression occurred within or adjacent to the CTV. The 1-year overall survival and local progression-free survival rates were 56% and 21%, respectively.
Conclusion: Despite the high response rate (64%) of this trial, there was a high incidence of in-field and marginal failure with this approach. Future studies combining BNCT with modalities other than radiation may be tried.
Keywords: boron neutron capture therapy; boronophenylalanine; head and neck cancer; image-guided intensity-modulated radiotherapy.
Conflict of interest statement
Ling-Wei Wang received grants from Heron Neutron Medical Corporation. The sponsors had no role in the design, execution, interpretation, or writing of the study.
Figures

References
-
- Sulman E.P., Schwartz D.L., Le T.T., Ang K.K., Morrison W.H., Rosenthal D.I., Ahamad A., Kies M., Glisson B., Weber R., et al. IMRT reirradiation of head and neck cancer-disease control and morbidity outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2009;73:399–409. doi: 10.1016/j.ijrobp.2008.04.021. - DOI - PubMed
-
- Ahlawat P., Rawat S., Kakria A., Devnani B., Wahi I.K., Simson D.K. Reirradiation with IMRT for recurrent head and neck cancer: A single-institutional report on disease control, survival, and toxicity. Rep. Pract. Oncol. Radiother. 2017;22:331–339. doi: 10.1016/j.rpor.2017.05.001. - DOI - PMC - PubMed
-
- Barth R.F., HVicente M., Harling O.K., Kiger W.S., Riley K.J., Binns P.J., Wagner F.M., Suzuki M., Aihara T., Kato I., et al. Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiat. Oncol. 2012;7:146. doi: 10.1186/1748-717X-7-146. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

