Genomic and Epigenetic Changes Drive Aberrant Skeletal Muscle Differentiation in Rhabdomyosarcoma
- PMID: 37345159
- PMCID: PMC10216238
- DOI: 10.3390/cancers15102823
Genomic and Epigenetic Changes Drive Aberrant Skeletal Muscle Differentiation in Rhabdomyosarcoma
Abstract
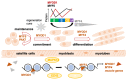
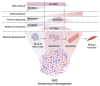
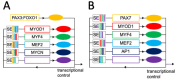
Rhabdomyosarcoma (RMS), the most common soft-tissue sarcoma in children and adolescents, represents an aberrant form of skeletal muscle differentiation. Both skeletal muscle development, as well as regeneration of adult skeletal muscle are governed by members of the myogenic family of regulatory transcription factors (MRFs), which are deployed in a highly controlled, multi-step, bidirectional process. Many aspects of this complex process are deregulated in RMS and contribute to tumorigenesis. Interconnected loops of super-enhancers, called core regulatory circuitries (CRCs), define aberrant muscle differentiation in RMS cells. The transcriptional regulation of MRF expression/activity takes a central role in the CRCs active in skeletal muscle and RMS. In PAX3::FOXO1 fusion-positive (PF+) RMS, CRCs maintain expression of the disease-driving fusion oncogene. Recent single-cell studies have revealed hierarchically organized subsets of cells within the RMS cell pool, which recapitulate developmental myogenesis and appear to drive malignancy. There is a large interest in exploiting the causes of aberrant muscle development in RMS to allow for terminal differentiation as a therapeutic strategy, for example, by interrupting MEK/ERK signaling or by interfering with the epigenetic machinery controlling CRCs. In this review, we provide an overview of the genetic and epigenetic framework of abnormal muscle differentiation in RMS, as it provides insights into fundamental mechanisms of RMS malignancy, its remarkable phenotypic diversity and, ultimately, opportunities for therapeutic intervention.
Keywords: differentiation; epigenetics; genomics; rhabdomyosarcoma; skeletal muscle.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the review; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the review.
Figures


References
-
- Rudzinski E.R., Kelsey A., Vokuhl C., Linardic C.M., Shipley J., Hettmer S., Koscielniak E., Hawkins D.S., Bisogno G. Pathology of childhood rhabdomyosarcoma: A consensus opinion document from the Children’s Oncology Group, European Paediatric Soft Tissue Sarcoma Study Group, and the Cooperative Weichteilsarkom Studiengruppe. Pediatr. Blood Cancer. 2021;68:e28798. doi: 10.1002/pbc.28798. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

