DNA methyltransferase inhibitor exposure-response: Challenges and opportunities
- PMID: 37345219
- PMCID: PMC10432879
- DOI: 10.1111/cts.13548
DNA methyltransferase inhibitor exposure-response: Challenges and opportunities
Abstract
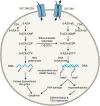
Although DNA methyltransferase inhibitors (DNMTis), such as azacitidine and decitabine, are used extensively in the treatment of myelodysplastic syndromes and acute myeloid leukemia, there remain unanswered questions about DNMTi's mechanism of action and predictors of clinical response. Because patients often remain on single-agent DNMTis or DNMTi-containing regimens for several months before knowing whether clinical benefit can be achieved, the development and clinical validation of response-predictive biomarkers represents an important unmet need in oncology. In this review, we will summarize the clinical studies that led to the approval of azacitidine and decitabine, as well as the real-world experience with these drugs. We will then focus on biomarker development for DNMTis-specifically, efforts at determining exposure-response relationships and challenges that remain impacting the broader clinical translation of these methods. We will highlight recent progress in liquid-chromatography tandem mass spectrometry technology that has allowed for the simultaneous measurement of decitabine genomic incorporation and global DNA methylation, which has significant potential as a mechanism-of-action based biomarker in patients on DNMTis. Last, we will cover important research questions that need to be addressed in order to optimize this potential biomarker for clinical use.
© 2023 The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Nicole M. Anders, Srinivasan Yegnasubramanian, and Michelle A. Rudek are inventors on the patent for Quantitative Determination of Nucleoside Analogue Drugs in Genomic DNA or RNA (US Patent 11,035,850 B2; expiration April 10, 2037). Amanda B. Kagan, Dominique A. Garrison, Nicole M. Anders, Jonathan A. Webster, and Sharyn D. Baker declare no competing interests for this work.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

