CCL24 regulates biliary inflammation and fibrosis in primary sclerosing cholangitis
- PMID: 37345655
- PMCID: PMC10371243
- DOI: 10.1172/jci.insight.162270
CCL24 regulates biliary inflammation and fibrosis in primary sclerosing cholangitis
Abstract
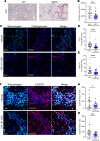
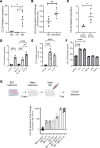
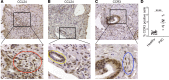
ˆCCL24 is a pro-fibrotic, pro-inflammatory chemokine expressed in several chronic fibrotic diseases. In the liver, CCL24 plays a role in fibrosis and inflammation, and blocking CCL24 led to reduced liver injury in experimental models. We studied the role of CCL24 in primary sclerosing cholangitis (PSC) and evaluated the potential therapeutic effect of blocking CCL24 in this disease. Multidrug resistance gene 2-knockout (Mdr2-/-) mice demonstrated CCL24 expression in liver macrophages and were used as a relevant experimental PSC model. CCL24-neutralizing monoclonal antibody, CM-101, significantly improved inflammation, fibrosis, and cholestasis-related markers in the biliary area. Moreover, using spatial transcriptomics, we observed reduced proliferation and senescence of cholangiocytes following CCL24 neutralization. Next, we demonstrated that CCL24 expression was elevated under pro-fibrotic conditions in primary human cholangiocytes and macrophages, and it induced proliferation of primary human hepatic stellate cells and cholangiocytes, which was attenuated following CCL24 inhibition. Correspondingly, CCL24 was found to be highly expressed in liver biopsies of patients with PSC. CCL24 serum levels correlated with Enhanced Liver Fibrosis score, most notably in patients with high alkaline phosphatase levels. These results suggest that blocking CCL24 may have a therapeutic effect in patients with PSC by reducing liver inflammation, fibrosis, and cholestasis.
Keywords: Chemokines; Fibrosis; Hepatology; Inflammation; Macrophages.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

