Activated cGAS/STING signaling elicits endothelial cell senescence in early diabetic retinopathy
- PMID: 37345657
- PMCID: PMC10371250
- DOI: 10.1172/jci.insight.168945
Activated cGAS/STING signaling elicits endothelial cell senescence in early diabetic retinopathy
Abstract
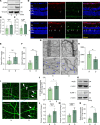
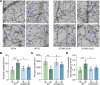
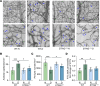
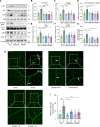
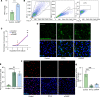
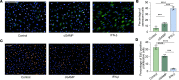
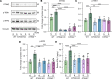
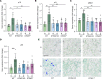
Diabetic retinopathy (DR) is a leading cause of blindness in working-age adults and remains an important public health issue worldwide. Here we demonstrate that the expression of stimulator of interferon genes (STING) is increased in patients with DR and animal models of diabetic eye disease. STING has been previously shown to regulate cell senescence and inflammation, key contributors to the development and progression of DR. To investigate the mechanism whereby STING contributes to the pathogenesis of DR, diabetes was induced in STING-KO mice and STINGGT (loss-of-function mutation) mice, and molecular alterations and pathological changes in the retina were characterized. We report that retinal endothelial cell senescence, inflammation, and capillary degeneration were all inhibited in STING-KO diabetic mice; these observations were independently corroborated in STINGGT mice. These protective effects resulted from the reduction in TBK1, IRF3, and NF-κB phosphorylation in the absence of STING. Collectively, our results suggest that targeting STING may be an effective therapy for the early prevention and treatment of DR.
Keywords: Cellular senescence; Ophthalmology; Retinopathy; Signal transduction.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

