Immune checkpoint activity regulates polycystic kidney disease progression
- PMID: 37345660
- PMCID: PMC10371237
- DOI: 10.1172/jci.insight.161318
Immune checkpoint activity regulates polycystic kidney disease progression
Abstract
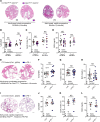
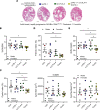
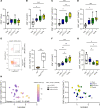
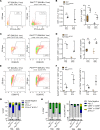
Innate and adaptive immune cells modulate the severity of autosomal dominant polycystic kidney disease (ADPKD), a common kidney disease with inadequate treatment options. ADPKD has parallels with cancer, in which immune checkpoint inhibitors have been shown to reactivate CD8+ T cells and slow tumor growth. We have previously shown that in PKD, CD8+ T cell loss worsens disease. This study used orthologous early-onset and adult-onset ADPKD models (Pkd1 p.R3277C) to evaluate the role of immune checkpoints in PKD. Flow cytometry of kidney cells showed increased levels of programmed cell death protein 1 (PD-1)/cytotoxic T lymphocyte associated protein 4 (CTLA-4) on T cells and programmed cell death ligand 1 (PD-L1)/CD80 on macrophages and epithelial cells in Pkd1RC/RC mice versus WT, paralleling disease severity. PD-L1/CD80 was also upregulated in ADPKD human cells and patient kidney tissue versus controls. Genetic PD-L1 loss or treatment with an anti-PD-1 antibody did not impact PKD severity in early-onset or adult-onset ADPKD models. However, treatment with anti-PD-1 plus anti-CTLA-4, blocking 2 immune checkpoints, improved PKD outcomes in adult-onset ADPKD mice; neither monotherapy altered PKD severity. Combination therapy resulted in increased kidney CD8+ T cell numbers/activation and decreased kidney regulatory T cell numbers correlative with PKD severity. Together, our data suggest that immune checkpoint activation is an important feature of and potential novel therapeutic target in ADPKD.
Keywords: Adaptive immunity; Monogenic diseases; Nephrology.
Figures



Comment in
-
Immune-checkpoint inhibition to slow cyst growth.Nat Rev Nephrol. 2023 Sep;19(9):541. doi: 10.1038/s41581-023-00751-8. Nat Rev Nephrol. 2023. PMID: 37524862 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

