Cloneable inorganic nanoparticles
- PMID: 37345851
- PMCID: PMC10334364
- DOI: 10.1039/d3cc01319g
Cloneable inorganic nanoparticles
Abstract
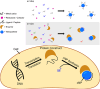
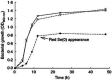
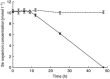
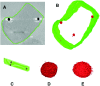
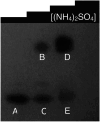
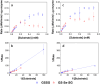
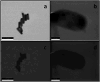
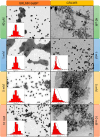
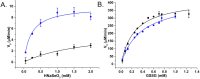
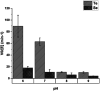
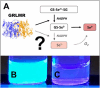
When a defined protein/peptide (or combinations thereof) control and define the synthesis of an inorganic nanoparticle, the result is a cloneable NanoParticle (cNP). This is because the protein sequence/structure/function is encoded in DNA, and therefore the physicochemical properties of the nanoparticle are also encoded in DNA. Thus the cloneable nanoparticle paradigm can be considered as an extension of the central dogma of molecular biology (e.g. DNA → mRNA → protein → cNP); modifications to the DNA encoding a cNP can modify the resulting properties of the cNP. Inorganic ion oxidoreductases (e.g., mercuric reductase, tellurite reductase, etc.) can select and reduce specific inorganic oxyanions and coordination complexes, creating zerovalent precipitates. Other proteins/peptides (often genetically concatenated to the parent oxidoreductase) serve as ligands, directing the size, shape, crystal structure and other properties of the nanoparticle. The DNA encoding a cNP can be recombinantly transferred into any organism. Ideally, this enables recombinant production of cNPs with the same defined physiochemical properties. Such cNPs are of interest for applications ranging from molecular imaging, bio-remediation, catalysis, and biomining. In this Feature Article we detail and define the cNP concept, and retrace the story of our creation of a cloneable Se NanoParticle (cSeNP). We also describe our more preliminary work that we expect to result in cloneable semiconductor quantum dots, cloneable Te nanoparticles, and other cNP formulations. We highlight the application of cNPs in cellular electron microscopy and compare this approach to other cloneable imaging contrast approaches.
Conflict of interest statement
There are no conflicts to declare.
Figures

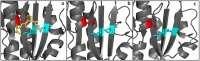



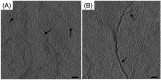









References
-
- Jogler C. Lin W. Meyerdierks A. Kube M. Katzmann E. Flies C. Pan Y. Amann R. Reinhardt R. Schüler D. Toward Cloning of the Magnetotactic Metagenome: Identification of Magnetosome Island Gene Clusters in Uncultivated Magnetotactic Bacteria from Different Aquatic Sediments. Appl. Environ. Microbiol. 2009;75(12):3972–3979. doi: 10.1128/AEM.02701-08. doi: 10.1128/AEM.02701-08. - DOI - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

