Clinical symptoms of SARS-CoV-2 breakthrough infection during the Omicron period in relation to baseline immune status and booster vaccination-A prospective multicentre cohort of health professionals (SURPRISE study)
- PMID: 37346094
- PMCID: PMC10279996
- DOI: 10.1111/irv.13167
Clinical symptoms of SARS-CoV-2 breakthrough infection during the Omicron period in relation to baseline immune status and booster vaccination-A prospective multicentre cohort of health professionals (SURPRISE study)
Abstract
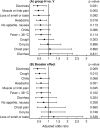
The effects of different types of pre-existing immunity on the frequency of clinical symptoms caused by the SARS-CoV-2 breakthrough infection were prospectively assessed in healthcare workers during the Omicron period. Among 518 participants, hybrid immunity was associated with symptom reduction for dizziness, muscle or limb pain and headache as compared to vaccination only. Moreover, the frequencies of dizziness, cough and muscle or limb pain were lower in participants who had received a booster vaccine dose. Thus, hybrid immunity appeared to be superior in preventing specific symptoms during breakthrough infection compared to vaccination alone. A booster vaccine dose conferred additional symptom reduction.
Keywords: Covid‐19; breakthrough infection; health professionals; symptoms; vaccine.
© 2023 The Authors. Influenza and Other Respiratory Viruses published by John Wiley & Sons Ltd.
Conflict of interest statement
No conflict of interest was declared.
Figures
References
-
- World Health Organization . Interim statement on hybrid immunity and increasing population seroprevalence rates (June 1, 2022). Accessed July 1, 2022. Available at: https://www.who.int/news/item/01-06-2022-interim-statement-on-hybrid-imm...
-
- Suarez Castillo M, Khaoua H, Courtejoie N. Vaccine‐induced and naturally‐acquired protection against omicron and Delta symptomatic infection and severe COVID‐19 outcomes, France, December 2021 to January 2022. Eurosurveillance. 2022;27. Available at:(16):2200250. doi: 10.2807/1560-7917.ES.2022.27.16.2200250. Accessed 16 June 2022 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

