The role of ion solvation in lithium mediated nitrogen reduction
- PMID: 37346742
- PMCID: PMC10281334
- DOI: 10.1039/d2ta07686a
The role of ion solvation in lithium mediated nitrogen reduction
Abstract
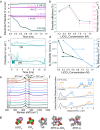
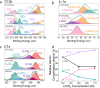
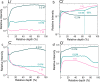
Since its verification in 2019, there have been numerous high-profile papers reporting improved efficiency of lithium-mediated electrochemical nitrogen reduction to make ammonia. However, the literature lacks any coherent investigation systematically linking bulk electrolyte properties to electrochemical performance and Solid Electrolyte Interphase (SEI) properties. In this study, we discover that the salt concentration has a remarkable effect on electrolyte stability: at concentrations of 0.6 M LiClO4 and above the electrode potential is stable for at least 12 hours at an applied current density of -2 mA cm-2 at ambient temperature and pressure. Conversely, at the lower concentrations explored in prior studies, the potential required to maintain a given N2 reduction current increased by 8 V within a period of 1 hour under the same conditions. The behaviour is linked more coordination of the salt anion and cation with increasing salt concentration in the electrolyte observed via Raman spectroscopy. Time of flight secondary ion mass spectrometry and X-ray photoelectron spectroscopy reveal a more inorganic, and therefore more stable, SEI layer is formed with increasing salt concentration. A drop in faradaic efficiency for nitrogen reduction is seen at concentrations higher than 0.6 M LiClO4, which is attributed to a combination of a decrease in nitrogen solubility and diffusivity as well as increased SEI conductivity as measured by electrochemical impedance spectroscopy.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Morlanés N. Katikaneni S. P. Paglieri S. N. et al., A technological roadmap to the ammonia energy economy: Current state and missing technologies. Chem. Eng. J. 2021;408:127310. doi: 10.1016/j.cej.2020.127310. - DOI
-
- Lazouski N. Schiffer Z. J. Williams K. et al., Understanding Continuous Lithium-Mediated Electrochemical Nitrogen Reduction. Joule. 2019;3:1127–1139. doi: 10.1016/j.joule.2019.02.003. - DOI
-
- Wang M. Kahn M. A. Mohsin I. et al., Can sustainable ammonia synthesis pathways compete with fossil-fuel based Haber–Bosch processes? Energy Environ. Sci. 2021;14(5):2535–2548. doi: 10.1039/D0EE03808C. - DOI
-
- Comer B. M. Fuentes P. Dimkpa C. O. et al., Prospects and Challenges for Solar Fertilizers. Joule. 2019;3:1578–1605. doi: 10.1016/j.joule.2019.05.001. - DOI
LinkOut - more resources
Full Text Sources
