Crucial Role for Lipoteichoic Acid Assembly in the Metabolic Versatility and Antibiotic Resistance of Staphylococcus aureus
- PMID: 37347167
- PMCID: PMC10353449
- DOI: 10.1128/iai.00550-22
Crucial Role for Lipoteichoic Acid Assembly in the Metabolic Versatility and Antibiotic Resistance of Staphylococcus aureus
Abstract
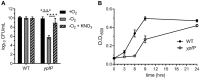
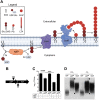
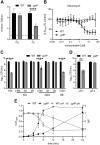
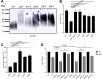
Staphylococcus aureus is a public health threat due to the prevalence of antibiotic resistance and the capacity of this organism to infect numerous organs in vertebrates. To generate energy needed to proliferate within tissues, S. aureus transitions between aerobic respiration and fermentation. Fermentation results in a distinct colony morphology called the small-colony variant (SCV) due to decreased membrane potential and ATP production. These traits promote increased resistance to aminoglycoside antibiotics. Consequently, SCVs are associated with persistent infections. We hypothesize that dedicated physiological pathways support fermentative growth of S. aureus that represent potential targets for treatment of resistant infections. Lipoteichoic acid (LTA) is an essential component of the Gram-positive cell envelope that functions to maintain ion homeostasis, resist osmotic stress, and regulate autolytic activity. Previous studies revealed that perturbation of LTA reduces viability of metabolically restricted S. aureus, but the mechanism by which LTA supports S. aureus metabolic versatility is unknown. Though LTA is essential, the enzyme that synthesizes the modified lipid anchor, YpfP, is dispensable. However, ypfP mutants produce altered LTA, leading to elongation of the polymer and decreased cell association. We demonstrate that viability of ypfP mutants is significantly reduced upon environmental and genetic induction of fermentation. This anaerobic viability defect correlates with decreased membrane potential and is restored upon cation supplementation. Additionally, ypfP suppressor mutants exhibiting restored anaerobic viability harbor compensatory mutations in the LTA biosynthetic pathway that restore membrane potential. Overall, these results demonstrate that LTA maintains membrane potential during fermentative proliferation and promotes S. aureus metabolic versatility.
Keywords: Staphylococcus aureus; cation; glycolipid anchor; ion homeostasis; lipoteichoic acid; membrane potential; metabolism; small-colony variant; ypfP.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK, Active Bacterial Core surveillance (ABCs) MRSA Investigarors . 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771. doi: 10.1001/jama.298.15.1763. - DOI - PubMed
-
- Kuehnert MJ, Kruszon-Moran D, Hill HA, McQuillan G, McAllister SK, Fosheim G, McDougal LK, Chaitram J, Jensen B, Fridkin SK, Killgore G, Tenover FC. 2006. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001–2002. J Infect Dis 193:172–179. doi: 10.1086/499632. - DOI - PubMed
-
- Schwerdt M, Neumann C, Schwartbeck B, Kampmeier S, Herzog S, Görlich D, Dübbers A, Große-Onnebrink J, Kessler C, Küster P, Schültingkemper H, Treffon J, Peters G, Kahl BC. 2018. Staphylococcus aureus in the airways of cystic fibrosis patients - a retrospective long-term study. Int J Med Microbiol 308:631–639. doi: 10.1016/j.ijmm.2018.02.003. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

