Evaluation of Ortho VITROS and Roche Elecsys S and NC Immunoassays for SARS-CoV-2 Serosurveillance Applications
- PMID: 37347180
- PMCID: PMC10434072
- DOI: 10.1128/spectrum.03234-22
Evaluation of Ortho VITROS and Roche Elecsys S and NC Immunoassays for SARS-CoV-2 Serosurveillance Applications
Abstract
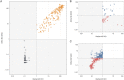
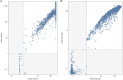
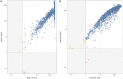
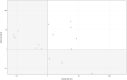
SARS-CoV-2 seroprevalence studies are instrumental in monitoring epidemic activity and require well-characterized, high-throughput assays, and appropriate testing algorithms. The U.S. Nationwide Blood Donor Seroprevalence Study performed monthly cross-sectional serological testing from July 2020 to December 2021, implementing evolving testing algorithms in response to changes in pandemic activity. With high vaccine uptake, anti-Spike (S) reactivity rates reached >80% by May 2021, and the study pivoted from reflex Roche anti-nucleocapsid (NC) testing of Ortho S-reactive specimens to parallel Ortho S/NC testing. We evaluated the performance of the Ortho NC assay as a replacement for the Roche NC assay and compared performance of parallel S/NC testing on both platforms. Qualitative and quantitative agreement of Ortho NC with Roche NC assays was evaluated on preselected S/NC concordant and discordant specimens. All 190 Ortho S+/Roche NC+ specimens were reactive on the Ortho NC assay; 34% of 367 Ortho S+/Roche NC- specimens collected prior to vaccine availability and 43% of 37 Ortho S-/Roche NC+ specimens were reactive on the Ortho NC assay. Performance of parallel S/NC testing using Ortho and Roche platforms was evaluated on 200 specimens collected in 2019 and 3,903 study specimens collected in 2021. All 200 pre-COVID-19 specimens tested negative on the four assays. Cross-platform agreement between Roche and Ortho platforms was 96.4% (3,769/3,903); most discordant results had reactivity close to the cutoffs on the alternate assays. These findings, and higher efficiency and throughput, support the use of parallel S/NC testing on either Roche or Ortho platforms for large serosurveillance studies. IMPORTANCE Seroprevalence studies like the U.S. Nationwide Blood Donor Seroprevalence Study (NBDS) have been critical in monitoring SARS-CoV-2 epidemic activity. These studies rely on serological assays to detect antibodies indicating prior infection. It is critical that the assays and testing algorithms used in seroprevalence studies have adequate performance (high sensitivity, high specificity, ability to discriminate vaccine-induced and infection-induced antibodies, etc.), as well as appropriate characteristics to support large-scale studies, such as high throughput and low cost. In this study we evaluated the performance of Ortho's anti-nucleocapsid assay as a replacement for the Roche anti-nucleocapsid assay and compared performance of parallel anti-spike and anti-nucleocapsid testing on both platforms. These data demonstrate similar performance of the Ortho and Roche anti-nucleocapsid assays and that parallel anti-spike and anti-nucleocapsid testing on either platform could be used for serosurveillance applications.
Keywords: COVID-19; SARS-CoV-2; antibodies; serology; serosurveillance; serosurvey.
Conflict of interest statement
The authors declare a conflict of interest. Vitalant Research Institute receives research funds and reagents for studies from Ortho Clinical Diagnostics and Roche.
Figures

Similar articles
-
Concordance of SARS-CoV-2 Antibody Results during a Period of Low Prevalence.mSphere. 2022 Oct 26;7(5):e0025722. doi: 10.1128/msphere.00257-22. Epub 2022 Sep 29. mSphere. 2022. PMID: 36173112 Free PMC article.
-
SARS-CoV-2 Antibody Testing in Health Care Workers: A Comparison of the Clinical Performance of Three Commercially Available Antibody Assays.Microbiol Spectr. 2021 Oct 31;9(2):e0039121. doi: 10.1128/Spectrum.00391-21. Epub 2021 Sep 29. Microbiol Spectr. 2021. PMID: 34585976 Free PMC article.
-
Comparison of SARS-CoV-2 seroprevalence estimates between commercial lab serum specimens and blood donor specimens, United States, September-December 2021.Microbiol Spectr. 2024 Aug 6;12(8):e0012324. doi: 10.1128/spectrum.00123-24. Epub 2024 Jun 13. Microbiol Spectr. 2024. PMID: 38869287 Free PMC article.
-
Performance of Elecsys Anti-SARS CoV-2 (Roche) and VIDAS Anti-SARS CoV-2 (Biomérieux) for SARS-CoV-2 Nucleocapsid and Spike Protein Antibody Detection.EJIFCC. 2022 Aug 8;33(2):159-165. eCollection 2022 Aug. EJIFCC. 2022. PMID: 36313907 Free PMC article. Review.
-
Technical considerations to development of serological tests for SARS-CoV-2.Talanta. 2021 Mar 1;224:121883. doi: 10.1016/j.talanta.2020.121883. Epub 2020 Nov 10. Talanta. 2021. PMID: 33379092 Free PMC article. Review.
Cited by
-
Reply to "Vaccination caused underestimation of cumulative incidence of SARS-CoV-2 infection".Microbiol Spectr. 2025 Feb 4;13(2):e0197924. doi: 10.1128/spectrum.01979-24. Epub 2024 Dec 16. Microbiol Spectr. 2025. PMID: 39679675 Free PMC article. No abstract available.
-
Detection of Nucleocapsid Antibodies Associated with Primary SARS-CoV-2 Infection in Unvaccinated and Vaccinated Blood Donors.Emerg Infect Dis. 2024 Aug;30(8):1621-1630. doi: 10.3201/eid3008.240659. Epub 2024 Jul 9. Emerg Infect Dis. 2024. PMID: 38981189 Free PMC article.
-
Proportions of US Blood Donors With Serological Evidence of Severe Acute Respiratory Syndrome Coronavirus 2 Infections Who Reported Survey-Based Diagnosed Infections During July 2020-December 2022.Open Forum Infect Dis. 2025 Apr 10;12(5):ofaf210. doi: 10.1093/ofid/ofaf210. eCollection 2025 May. Open Forum Infect Dis. 2025. PMID: 40302721 Free PMC article.
-
Long-Term Symptoms Associated With SARS-CoV-2 Infection Among Blood Donors.JAMA Netw Open. 2024 Apr 1;7(4):e245611. doi: 10.1001/jamanetworkopen.2024.5611. JAMA Netw Open. 2024. PMID: 38587842 Free PMC article.
-
Prevalence of SARS-CoV-2 infection among US blood donors by industry, May-December 2021.Am J Ind Med. 2024 Feb;67(2):169-173. doi: 10.1002/ajim.23552. Epub 2023 Dec 4. Am J Ind Med. 2024. PMID: 38047323 Free PMC article.
References
-
- Stringhini S, Wisniak A, Piumatti G, Azman AS, Lauer SA, Baysson H, De Ridder D, Petrovic D, Schrempft S, Marcus K, Yerly S, Arm Vernez I, Keiser O, Hurst S, Posfay-Barbe KM, Trono D, Pittet D, Getaz L, Chappuis F, Eckerle I, Vuilleumier N, Meyer B, Flahault A, Kaiser L, Guessous I. 2020. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet 396:313–319. doi:10.1016/S0140-6736(20)31304-0. - DOI - PMC - PubMed
-
- Pollan M, Perez-Gomez B, Pastor-Barriuso R, Oteo J, Hernan MA, Perez-Olmeda M, Sanmartin JL, Fernandez-Garcia A, Cruz I, Fernandez de Larrea N, Molina M, Rodriguez-Cabrera F, Martin M, Merino-Amador P, Leon Paniagua J, Munoz-Montalvo JF, Blanco F, Yotti R, Group E-CS . 2020. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet 396:535–544. doi:10.1016/S0140-6736(20)31483-5. - DOI - PMC - PubMed
-
- Rosenberg ES, Tesoriero JM, Rosenthal EM, Chung R, Barranco MA, Styer LM, Parker MM, John Leung SY, Morne JE, Greene D, Holtgrave DR, Hoefer D, Kumar J, Udo T, Hutton B, Zucker HA. 2020. Cumulative incidence and diagnosis of SARS-CoV-2 infection in New York. Ann Epidemiol 48:23–29 e4. doi:10.1016/j.annepidem.2020.06.004. - DOI - PMC - PubMed
-
- Bobrovitz N, Arora RK, Cao C, Boucher E, Liu M, Donnici C, Yanes-Lane M, Whelan M, Perlman-Arrow S, Chen J, Rahim H, Ilincic N, Segal M, Duarte N, Van Wyk J, Yan T, Atmaja A, Rocco S, Joseph A, Penny L, Clifton DA, Williamson T, Yansouni CP, Evans TG, Chevrier J, Papenburg J, Cheng MP. 2021. Global seroprevalence of SARS-CoV-2 antibodies: a systematic review and meta-analysis. PLoS One 16:e0252617. doi:10.1371/journal.pone.0252617. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

