Cefiderocol Treatment for Patients with Multidrug- and Carbapenem-Resistant Pseudomonas aeruginosa Infections in the Compassionate Use Program
- PMID: 37347188
- PMCID: PMC10353454
- DOI: 10.1128/aac.00194-23
Cefiderocol Treatment for Patients with Multidrug- and Carbapenem-Resistant Pseudomonas aeruginosa Infections in the Compassionate Use Program
Abstract
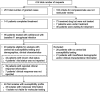
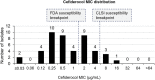
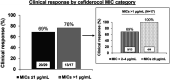
Cefiderocol is an option for infections caused by multidrug-resistant Pseudomonas aeruginosa, but its in vitro activity against these isolates and its clinical effectiveness for isolates with MICs of >1 μg/mL is unclear. We investigated the in vitro activity of cefiderocol against P. aeruginosa isolates collected from patients treated with cefiderocol through the compassionate use program and assessed physician-reported clinical response and 28-day all-cause mortality by cefiderocol MIC values. P. aeruginosa isolates underwent susceptibility testing to cefiderocol and comparator agents by using reference broth microdilution. U.S. Food and Drug Administration (FDA; susceptible, ≤1 μg/mL) and Clinical and Laboratory Standards Institute (CLSI; susceptible, ≤4 μg/mL) cefiderocol breakpoints were applied. Additionally, molecular characterization of β-lactamase genes was performed. Clinical response and vital status were reported by treating physicians. Forty-six patients with P. aeruginosa infections were evaluated. Twenty-nine (63%) and 42 (91%) isolates were susceptible to cefiderocol using FDA and CLSI breakpoints, respectively. Thirty-seven (80%) and 32 (70%) isolates were not susceptible to ceftolozane-tazobactam and ceftazidime-avibactam, respectively. The clinical response rate was 69% (20/29) with a cefiderocol MIC of ≤1 μg/mL, 69% (9/13) with a cefiderocol MIC of 2 to 4 μg/mL, and 100% (4/4) with an MIC of ≥8 μg/mL, while day 28 all-cause mortality rates were 23% (6/26; MIC ≤ 1 μg/mL), 33% (4/12; MIC, 2 to 4 μg/mL), and 0% (0/4; MIC ≥8 μg/mL), respectively. Cefiderocol was active in vitro against most P. aeruginosa isolated from patients with limited or no alternative therapies. Patients with cefiderocol MICs of 2 to 4 μg/mL did not have significantly worse outcomes than those with MICs of ≤1 μg/mL.
Keywords: carbapenem-resistant Pseudomonas aeruginosa; cefiderocol; clinical response; compassionate use; susceptibility breakpoint.
Conflict of interest statement
The authors declare a conflict of interest. M.J.S. had received consulting fees from Shionogi, participates in an Independent Data Monitoring Committee for AbbVie, and has received research funding through his institution from Merck, bioMérieux, SNIPR Biome, Affinity Biosensors, and Selux Diagnostics. He is also a Member of the CLSI Subcommittee on Antimicrobial Susceptibility Testing and Co-Chair of its Breakpoint Working Group. P.J.S. has received consulting fees from Shionogi. Outside of the submitted work, she reports grants and personal fees from OpGen Inc., bioMérieux, and BD Diagnostics, grants from Affinity Biosensors and Qiagen; and personal fees from GeneCapture, and Entasis. She is a voting Member of the CLSI Subcommittee on Antimicrobial Susceptibility Testing. C.M.S., Y.Y., T.D.N., S.P. are employees of Shionogi.
Figures
References
-
- World Health Organization Regional Office for Europe, European Centre for Disease Prevention and Control. 2022. Antimicrobial resistance surveillance Europe. https://www.ecdc.europa.eu/sites/default/files/documents/Joint-WHO-ECDC-.... Retrieved 3 October 2022.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

