Autoantibodies neutralizing type I IFNs underlie West Nile virus encephalitis in ∼40% of patients
- PMID: 37347462
- PMCID: PMC10287549
- DOI: 10.1084/jem.20230661
Autoantibodies neutralizing type I IFNs underlie West Nile virus encephalitis in ∼40% of patients
Abstract
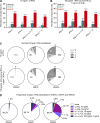
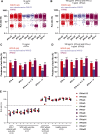
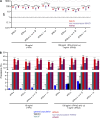
Mosquito-borne West Nile virus (WNV) infection is benign in most individuals but can cause encephalitis in <1% of infected individuals. We show that ∼35% of patients hospitalized for WNV disease (WNVD) in six independent cohorts from the EU and USA carry auto-Abs neutralizing IFN-α and/or -ω. The prevalence of these antibodies is highest in patients with encephalitis (∼40%), and that in individuals with silent WNV infection is as low as that in the general population. The odds ratios for WNVD in individuals with these auto-Abs relative to those without them in the general population range from 19.0 (95% CI 15.0-24.0, P value <10-15) for auto-Abs neutralizing only 100 pg/ml IFN-α and/or IFN-ω to 127.4 (CI 87.1-186.4, P value <10-15) for auto-Abs neutralizing both IFN-α and IFN-ω at a concentration of 10 ng/ml. These antibodies block the protective effect of IFN-α in Vero cells infected with WNV in vitro. Auto-Abs neutralizing IFN-α and/or IFN-ω underlie ∼40% of cases of WNV encephalitis.
© 2023 Gervais et al.
Conflict of interest statement
Disclosures: Q. Philippot reported grants from Assistance Publique Hopitaux de Paris, Fondation Bettencourt Schueller, ARS Ile de France, and personal fees from Gilead outside the submitted work. M.S. Diamond reported personal fees from Topspin Therapeutics outside the submitted work. J.-L. Casanova reported a patent to PCT/US2021/042741 pending. No other disclosures were reported.
Figures









References
-
- Alotaibi, F., Alharbi N.K., Rosen L.B., Asiri A.Y., Assiri A.M., Balkhy H.H., Al Jeraisy M., Mandourah Y., AlJohani S., Al Harbi S., et al. . 2023. Type I interferon autoantibodies in hospitalized patients with Middle East respiratory syndrome and association with outcomes and treatment effect of interferon beta-1b in MIRACLE clinical trial. Influenza Other Respir. Viruses. 17:e13116. 10.1111/irv.13116 - DOI - PMC - PubMed
-
- Barzon, L., Pacenti M., Montarsi F., Fornasiero D., Gobbo F., Quaranta E., Monne I., Fusaro A., Volpe A., Sinigaglia A., et al. . 2022. Rapid spread of a new West Nile virus lineage 1 associated with increased risk of neuroinvasive disease during a large outbreak in northern Italy, 2022: One Health analysis. J. Trav. Med. taac125. 10.1093/jtm/taac125 - DOI - PMC - PubMed
-
- Bastard, P., Gervais A., Le Voyer T., Rosain J., Philippot Q., Manry J., Michailidis E., Hoffmann H.-H., Eto S., Garcia-Prat M., et al. . 2021a. Autoantibodies neutralizing type I IFNs are present in ∼4% of uninfected individuals over 70 years old and account for ∼20% of COVID-19 deaths. Sci. Immunol. 6:eabl4340. 10.1126/sciimmunol.abl4340 - DOI - PMC - PubMed
-
- Bastard, P., Michailidis E., Hoffmann H.-H., Chbihi M., Le Voyer T., Rosain J., Philippot Q., Seeleuthner Y., Gervais A., Materna M., et al. . 2021b. Auto-antibodies to type I IFNs can underlie adverse reactions to yellow fever live attenuated vaccine. J. Exp. Med. 218:e20202486. 10.1084/jem.20202486 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01AI088364/NH/NIH HHS/United States
- R01 AI073755/AI/NIAID NIH HHS/United States
- R01 AI091707/AI/NIAID NIH HHS/United States
- R21 AI160576/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 DK122790/DK/NIDDK NIH HHS/United States
- R01 AI088364/AI/NIAID NIH HHS/United States
- R01 AI091816/AI/NIAID NIH HHS/United States
- R01 AI163029/AI/NIAID NIH HHS/United States
- UL1 TR001866/TR/NCATS NIH HHS/United States
- R01 AI124690/AI/NIAID NIH HHS/United States
- U19 AI089992/AI/NIAID NIH HHS/United States
- R01 AI127564/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

