Highly Pathogenic Avian Influenza Virus (H5N1) Clade 2.3.4.4b Introduced by Wild Birds, China, 2021
- PMID: 37347504
- PMCID: PMC10310395
- DOI: 10.3201/eid2907.221149
Highly Pathogenic Avian Influenza Virus (H5N1) Clade 2.3.4.4b Introduced by Wild Birds, China, 2021
Abstract
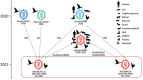
Highly pathogenic avian influenza (HPAI) subtype H5N1 clade 2.3.4.4b virus has spread globally, causing unprecedented large-scale avian influenza outbreaks since 2020. In 2021, we isolated 17 highly pathogenic avian influenza H5N1 viruses from wild birds in China. To determine virus origin, we genetically analyzed 1,529 clade 2.3.4.4b H5N1 viruses reported globally since October 2020 and found that they formed 35 genotypes. The 17 viruses belonged to genotypes G07, which originated from eastern Asia, and G10, which originated from Russia. The viruses were moderately pathogenic in mice but were highly lethal in ducks. The viruses were in the same antigenic cluster as the current vaccine strain (H5-Re14) used in China. In chickens, the H5/H7 trivalent vaccine provided complete protection against clade 2.3.4.4b H5N1 virus challenge. Our data indicate that vaccination is an effective strategy for preventing and controlling the globally prevalent clade 2.3.4.4b H5N1 virus.
Keywords: China; H5N1; antigenic property; clade 2.3.4.4b; influenza; influenza virus; phylogeny; protective efficacy; virulence; viruses; wild birds.
Figures


References
-
- World Health Organization/World Organisation of Animal Health/Food and Agriculture Organization of the United Nations/H5N1 Evolution Working Group. Continued evolution of highly pathogenic avian influenza A (H5N1): updated nomenclature. Influenza Other Respir Viruses. 2012;6:1–5. 10.1111/j.1750-2659.2011.00298.x - DOI - PMC - PubMed
-
- World Organisation for Animal Health. Latest animal disease events [cited 2022 May 3]. https://wahis.woah.org/#/home
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

